0321
Cortical ß-amyloid plaque load detection using QSM in Alzheimer’s patients at 9.4T1Department for Biomedical Magnetic Resonance, Eberhard Karl’s University and University Hospital, Tuebingen, Germany, 2Department for High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetic, Tuebingen, Germany, 3Department for High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 4German Center for Neurodegenerative Diseases (DZNE), Tuebingen, Germany, 5Section for Dementia Research, Hertie Institute for Clinical Brain Research and Department of Psychiatry and Psychotherapy, Tuebingen, Germany, 6Department for Biomedical Magnetic Resonance, Eberhard Karl’s University, Tuebingen and University Hospital, Tuebingen, Germany
Synopsis
Beta-amyloid (Aβ) plaques are characteristic of Alzheimer’s Disease (AD) brain and cause effects which can be detected by QSM. It has been shown that cortical plaque-load could be used to distinguish AD patients from healthy controls (HC) using ultra-high spatial resolution QSM at ultra-high-field (9.4 and 14.1T), in-vivo and ex-vivo. We aimed to extend these observations to a larger cohort of patients and controls at two different spatial resolutions. We found a significative (p<0.05) increase in plaque-load in AD compared to HC at both resolutions. Interestingly, some cortical regions also showed greater (p<0.05) diamagnetic effects in AD compared to HC.
PURPOSE
Beta amyloid (Aβ) plaques are characteristic of Alzheimer’s Disease (AD) brain and cause effects which can be detected by QSM1,2,3. Previously, it was found that the cortical plaque load can be used as a means to distinguish between AD patients and healthy controls (HC) using QSM at ultra-high spatial resolution in vivo and ex vivo, at ultra-high field (9.4 and 14.1T)1. The first aim of this study was to extend these observations to a larger cohort of patients and controls subjects. We also investigated the possibility to determine the plaque load at different spatial resolutions. For this purpose, results obtained from an ultra-high resolution single echo GRE sequence were compared with results obtained from a multi-echo GRE sequence with a more coarse spatial resolution in the same cortical regions of interest (ROI) from the Harvard-Oxford Cortical Atlas. We also investigated the diamagnetic effects in the same cortical ROIs as a complementary means to distinguish between AD and HC as both spatial resolutions.METHODS
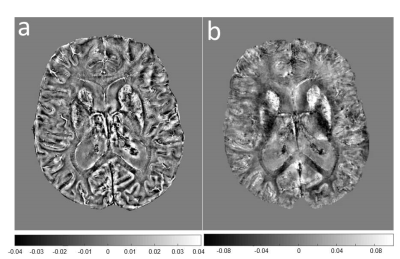
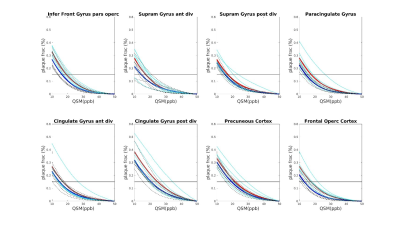
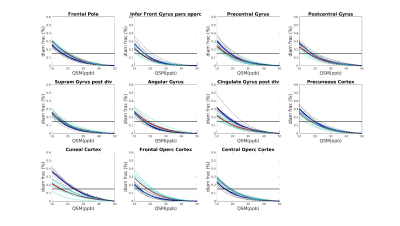
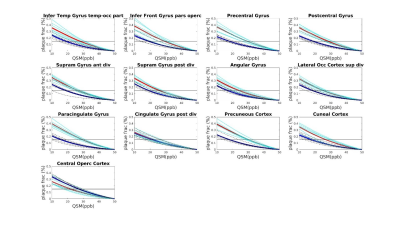
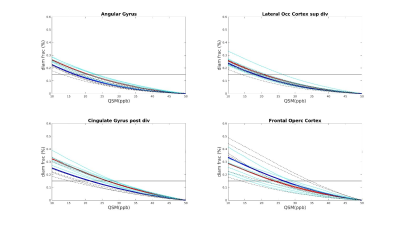
Seven AD patients and 7 healthy subjects, aged between 63 and 85, underwent scanning examinations in a 9.4T MRI whole body human research system (Siemens medical Systems, Erlangen, Germany). A custom-built 16-channel transmit (Tx) and 31-channel receive (Rx) RF-coil-array optimized for homogeneity and signal-to-noise ratio (SNR) inside the human head was used for transmission and detection of the induced signal4. Magnetization Prepared 2 Rapid Gradient Echo (MP2RAGE) images were acquired for definition of common cortical ROIs: inversion times = TI1/TI2 = 900/3500ms; TE=2.3ms, α = 4°/6°; read-out TR = 6ms; 800µm3 isotropic voxel size, matrix size=256x256x192, FOV = 205x205x154mm3, TA = 8min and 53s. A partial-coverage, flow-compensated, single-echo 3D GRE with weighted averaging of phase-encoding steps for ultra-high resolution imaging5, was acquired for QSM calculation (voxel size = 132x132x610µm3, field of view (FOV) = 180x135x12.8mm3, repetition time/echo time/flip angle: TR/TE/α = 24/16.5ms/8°, acquisition time (TA) = 14.75min). A multi-echo (N=5) 3D GRE sequence (0.375x0.375x0.8)mm3 voxel size, TR=35ms; TE=6 to 30ms in steps of 6ms, acquisition time = 9min, was acquired for lower resolution QSM calculation. Due to the reduced size of the ultra-high resolution maps, common ROIs across all subjects had to be defined for the analysis. The fraction of plaques and diamagnetic effects (“plaque frac” and “diam frac”, respectively) were then computed in 16 common ROIs as the number of paramagnetic (and diamagnetic) pixels divided by the number of pixels of the ROI, for different QSM thresholds (ranging from 10 to 50ppb, to exclude the contributions from the veins), at both resolutions. QSM was computed using the iLSQR-method (STI suite toolbox (http://people.duke.edu/~cl160/)), Laplacian-based unwrapping algorithm as implemented in MEDI toolbox (http://pre.weill.cornell.edu/mri/pages/qsm.html) at higher resolution and using the STI toolbox (http://people.duke.edu/~cl160/) at lower resolution. RESHARP algorithm was used for the background removal with slight different optimization parameter at different resolutions (Tikhonov = 10-3, kernel size=1.22mm at higher resolution, Tikhonov = 10-12 and kernel size=1.6mm at lower resolution).RESULTS
A significant difference (p<0.05) was found in the plaque fraction (paramagnetic effects) in AD patients compared to HC in 8 ROIs out of 16 common ROIs in ultra-high resolution susceptibility maps. Six of these ROIs were also found at the lower resolution maps. In addition, five other showed a significant difference (p<0.05) at the lower spatial resolution. Also, four of the ROIs with significant difference in plaque fraction in ultra-high resolution maps were also found significantly different in diamagnetic effects, whilst 7 ROIs only showed differences (p<0.05) in diamagnetic effects. Eleven cases out of 16 showed a significant difference between AD and HC in diamagnetic fraction at lower resolution. Three of these cases were in common with high-resolution maps and only one showed higher p values compared to ultra-high spatial resolution maps. Whilst, when comparing the paramagnetic fractions detected at different resolution multi-echo maps always showed higher significance. Results are resumed in table 1.DISCUSSION AND CONCLUSION
Ultra-high spatial resolution allows the detection of local changes compared to the global effect achieved using a lower spatial resolution. This study confirms that it is possible to distinguish between AD and HC using QSM at ultra-high field strength based on plaque load detection. The stronger effects observed at lower spatial resolution could be explained by the coarse localization of the plaque effects that may cause the presence of false positives in the plaque load detection. On the other hand, ultra-high resolution maps were critical to accurately define the spatial distribution of the plaques and the regions of interest for the analysis. Interestingly, many cortical regions also showed greater diamagnetic effects with distinct profiles in the two groups. In general, plaque fraction was higher in AD patients compared to HC, whilst diamagnetic effects were less straightforward to interpret.Acknowledgements
We gratefully acknowledge the EU-LACH Grant #16/T01-0118References
1. Tuzzi, E., Balla, D. Z., Loureiro, J. R., Neumann, M., Laske, C., Pohmann, R., ... & Hagberg, G. E. (2020). Ultra-High Field MRI in Alzheimer’s Disease: Effective Transverse Relaxation Rate and Quantitative Susceptibility Mapping of Human Brain In Vivo and Ex Vivo Compared to Histology. Journal of Alzheimer's Disease, (Preprint), 1-19.
2. Tiepolt S, Schafer A, Rullmann M, Roggenhofer E, Netherlands Brain B, Gertz HJ, Schroeter ML, Patt M, Bazin PL, Jochimsen TH, Turner R, Sabri O, Barthel H (2018) Quantitative susceptibility mapping of amyloid-beta aggregates in Alzheimer’s disease with 7T MR. J Alzheimers Dis 64, 393-404.
3. Chen, L., Soldan, A., Oishi, K., Faria, A., Zhu, Y., Albert, M., ... & Li, X. (2020). Quantitative susceptibility mapping of brain iron and β-amyloid in MRI and PET relating to cognitive performance in cognitively normal older adults. Radiology, 201603.
4. Shajan G, Kozlov M, Hoffmann J, Turner R, Scheffler K, Pohmann R (2014) A 16-channel dual-row transmit array in combination with a 31-element receive array for human brain imaging at 9.4 T. Magn Reson Med 71, 870-879.
5. Budde J, Shajan G, Scheffler K, Pohmann R (2014) Ultra-high resolution imaging of the human brain using acquisition-weighted imaging at 9.4T. Neuroimage 86, 592- 598.
Figures