0319
Characterization of Arterial and Portal Venous Contributions to Metabolic Imaging of the Human Liver Using Hyperpolarized 13C-pyruvate MRI
Philip Meng-en Lee1, Jeremy W Gordon1, Zhen J Wang1, Zihan Zhu1, Hsin-Yu Chen1, Pamela N Munster2, Rahul Aggarwal2, Daniel B Vigneron1, and Michael A Ohliger1
1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Analysis of the delivery and metabolism of hyperpolarized 13C pyruvate within the liver is complicated by the organ’s unique dual blood supply. Distinguishing the hepatic arterial and portal venous contributions of pyruvate delivery would improve real-time acquisition triggering and kinetic modeling. Three healthy subjects and two metastatic cancer subjects underwent hyperpolarized 13C MRI on a clinical 3 T MR scanner. We observed differential arrival of [1-13C]pyruvate signal in the aorta, inferior vena cava, portal vein, healthy liver, and metastases matching physiologic expectations. Consistencies were observed within subject groups, which is crucial for accurate timing and modelling of metabolic hyperpolarized signals.
Introduction
Hyperpolarized (HP) 13C MRI enables in vivo quantitative dynamic imaging of enzyme-catalyzed cellular metabolism, with clinical applications in prostate, brain, heart, and kidney.1–4 However, the unique dual blood supply of the liver (via the hepatic artery and portal vein) complicates quantitative analyses of both the delivery of injected HP substrates and the timing of subsequent observed metabolism. Decomposing the pyruvate delivery into the contributions of each blood vessel would allow for improved real-time acquisition triggering and kinetic modeling accuracy by providing perfusion information. Here, we investigate the distinct vascular contributions in the human liver and assess the effects on observed HP 13C signals in the liver.Methods
Three healthy subjects (Healthy #1-3), one subject with metastatic pancreatic cancer (Cancer #1), and one subject with metastatic breast cancer (Cancer #2) underwent HP 13C MRI on a clinical 3 T MR scanner with a 1-, 8-, or 16-channel surface coil/clamshell transmitter combination with an injection of 0.43 mL/kg of 250 mM HP [1-13C]pyruvate. Axial T1-weighted spoiled gradient-echo anatomical scans were acquired for blood vessel registration. For Healthy #3, spectroscopic data were acquired using a single-slice 2D echoplanar spectroscopic imaging (EPSI) sequence. Spectroscopic data were processed using a tensor rank truncation pipeline.5 For all other subjects, [1-13C]pyruvate, and downstream metabolites [1-13C]lactate, [1-13C]alanine, and [13C]bicarbonate signals were acquired using a specialized multi-slice 13C echo planar imaging acquisition with constant flip angles (38.5° for pyruvate and 68.3° for downstream metabolites, flip angles chosen to match the equivalent magnetization of 2D EPSI scans).2 The data was phase corrected and then Fourier transformed. Multi-channel data were pre-whitened and then coil combined using pyruvate to estimate the coil weights.6 All scans had a total scan time of 1 minute with a temporal resolution of 3 s and in-plane voxels of 1.0×1.0 to 2.0×2.0 cm, slice thicknesses of 2.0-3.0 cm, and 3-5 slices (single slice for Healthy #3).Results and Discussion
Dynamic curves of total 13C signal were obtained for voxels in the aorta, the inferior vena cava (IVC), the portal vein (PV), healthy liver (or normal appearing liver in cancer subjects), and liver metastasis. The total 13C dynamic curves for the healthy subjects and cancer subjects are shown in Figure 1 and Figure 2, respectively. The peak arrival times from the start of acquisition for each anatomical structure and subject are shown in Figure 3.Overall, we observed a differential arrival of [1-13C]pyruvate signal, first in the aorta followed by the IVC, PV, and lastly the healthy liver and liver metastasis, matching physiologic expectations as the IVC rapidly receives venous return from the kidneys while the PV is delayed as it receives blood that passes first through the gastrointestinal tract. The healthy subjects showed a consistent peak arrival time relative to the aorta in the IVC (5.1-8.2 s), in the PV (7.3-12.4 s), and healthy liver (12.3-16.9 s). A different pattern was observed in both cancer subjects; a shorter peak arrival time relative to the aorta was observed in the IVC (0.7-1.4 s), in the PV (3.4-4.5 s), and normal appearing liver (4.5-6 s). This is likely due to neovascularization of the hepatic tumor and a generally more disorganized vascular supply to these growths.
Conclusions
Distinct vascular contributions can be observed from the liver’s dual blood supply, despite the limited lifetime of the HP signal. For optimal HP 13C-pyruvate MRI, the timing of image acquisition is important. These results will help refine models of hyperpolarized liver metabolism and tailor real-time acquisition strategies, such as bolus tracking,7 to optimize HP 13C imaging of the liver.Acknowledgements
This work was supported by NIH grants NIDDK 5R01DK115987 and P41 EB013598.References
- Chen HY, Aggarwal R, Bok RA, et al. Hyperpolarized 13C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: a clinical feasibility study. Prostate Cancer Prostatic Dis. 2020;23(2):269-276. doi:10.1038/s41391-019-0180-z
- Gordon JW, Chen HY, Autry A, et al. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med. 2019;81(4):2702-2709. doi:10.1002/mrm.27549
- Cunningham CH, Lau JYC, Chen AP, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016;119(11):1177-1182. doi:10.1161/CIRCRESAHA.116.309769
- Tang S, Bok R, Qin H, et al. A metabolite-specific 3D stack-of-spiral bSSFP sequence for improved lactate imaging in hyperpolarized [1-13C]pyruvate studies on a 3T clinical scanner. Magn Reson Med. 2020;84(3):1113-1125. doi:10.1002/mrm.28204
- Chen HY, Autry AW, Brender JR, et al. Tensor image enhancement and optimal multichannel receiver combination analyses for human hyperpolarized 13C MRSI. Magn Reson Med. 2020;84(6):3351-3365. doi:10.1002/mrm.28328
- Zhu Z, Zhu X, Ohliger MA, et al. Coil combination methods for multi-channel hyperpolarized 13 C imaging data from human studies. J Magn Reson. 2019;301:73-79. doi:10.1016/j.jmr.2019.01.015
- Tang S, Milshteyn E, Reed G, et al. A regional bolus tracking and real-time B1 calibration method for. Magn Reson Med. 2019;81(2):839-851. doi:10.1002/mrm.27391
Figures
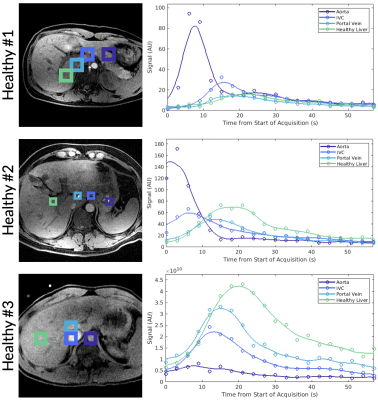
Figure 1. Total HP 13C signal plotted over time for selected tissue voxels in three healthy human subjects with ROIs labeled in the adjacent anatomical image. Spatial resolution varied between acquisitions.
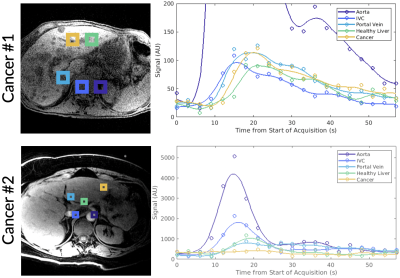
Figure 2. Total 13C signals plotted over time for selected tissue voxels in a metastatic pancreatic cancer patient (Cancer #1) and in a metastatic breast cancer patient (Cancer #2) with ROIs labeled in the adjacent anatomical image. Spatial resolution varied between acquisitions.

Figure 3. Peak arrival times of total 13C signal for each subject (t = 0 coincides with the start of the acquisition). The tumor for Cancer #2 fell within a saturation band and no signal was acquired (marked with a diamond).