0318
Repeatability of Liver Apparent Diffusion Coefficient Measurement Using Free-Breathing Diffusion-Weighted Propeller Echo-Planar Imaging1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Automatic Control Engineering, Feng Chia University, Taichung, Taiwan, 3Department of Medical Imaging, China Medical University Hsinchu Hospital, Hsinchu, Taiwan, 4Department of Radiology, School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan, 5Department of Medical Imaging, China Medical University Hospital, Taichung, Taiwan, 6Department of Electrical Engineering, National Taiwan University, Taipei, Taiwan, 7Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan
Synopsis
The current limitations of liver DWI mainly relate to the image quality and the repeatability/reproducibility of ADC measurement using single-shot DW echo-planar imaging (DW-EPI). The discrepancy of ADC measurement can substantially cause difficulty in cross-sectional or longitudinal liver DW-EPI. A preliminary study reported that a free-breathing liver DW-Propeller-EPI technique can provide superior image quality to conventional liver DW-EPI methods. In this study, we further improved the robustness of free-breathing liver DW-Propeller-EPI by incorporating velocity-compensation (VC) diffusion gradient into data acquisition, and then evaluated the repeatability of liver ADC measurement for free-breathing DW-Propeller-EPI by comparing to three routine liver DW-EPI methods.
INTRODUCTION
Diffusion-weighted imaging (DWI) is increasingly implemented into routine liver MRI for detecting lesion1,2, and potentially assessing liver fibrosis and cirrhosis by measuring apparent diffusion coefficient (ADC)3-5. However, the current limitations of liver DWI mainly relate to the image quality and the repeatability/reproducibility of ADC measurement6. First, the use of single-shot DW echo-planar imaging (DW-EPI) for liver DWI acquisition leads to geometric distortion and image blurring7, and thus suboptimal for clinical applications8-10. Second, the respiratory motion prominently escalates the difficulty in implementing liver DW-EPI11,12. The irregular respiration rate may also affect the data integrity when using respiratory triggering, and some patient populations may also have difficulty in holding their breathing13. In light of this, we have previously proposed a free-breathing liver DW-Propeller-EPI technique that can provide superior image quality to conventional liver DW-EPI acquisition14. In this study, we further improved the robustness of free-breathing liver DW-Propeller-EPI by incorporating velocity-compensation (VC) diffusion gradient into data acquisition, and then evaluated the repeatability of liver ADC measurement for free-breathing DW-Propeller-EPI.METHODS
Pulse Sequence Design: During diffusion probing period, the respiratory motion can induce substantial phase shift, and cause signal loss in liver DWI. To reduce the signal loss of free-breathing liver DWI, VC diffusion preparation gradient was incorporated into DW-Propeller-EPI pulse sequence (Fig.1), for eliminating all phase sensitivity to the first-moment velocity motion11.Data Collection and Reconstruction: Liver DWI data were acquired from 22 subjects at a 1.5T MRI (Explorer, GE Healthcare) using a 12-channel phase-array body coil. The protocol included:
1) Breath-hold axial fast gradient-echo (FGRE) imaging (TR=170ms, TE=2.1ms, NEX=1, matrix=224×192, FOV=360mm, scantime=0:18);
2) Breath-hold DW-EPI (TR=1600ms, TE=78.9ms, NEX=3, scantime=0:18);
3) Free-breathing DW-EPI (TR=6000ms, TE=78.9ms, NEX=4, scantime=1:45);
4) Respiratory-triggered DW-EPI (TR=5000~7000ms, TE=78.9ms, NEX=4, scantime~3:00); and
5) Free-breathing DW-Propeller-EPI (TR=3500ms, TE=88.1ms, NEX=1, blade size=128×32, rotating angle=15°, 24 blades for 360° k-space coverage, scantime=5:40).
All diffusion sequences were acquired with 128×128 matrix size, 8mm slice thickness, 20 slices for whole liver coverage, 360mm FOV, and three orthogonal diffusion directions with b-values of 500 s/mm2. For the purpose of evaluating repeatability in ADC measurement, all diffusion sequences were performed twice on each subject. The raw data of DW-Propeller-EPI were transferred to a workstation for Nyquist ghost correction and image reconstruction14, and the reconstructed data were interpolated to 256×256 resolution for matching the image size of scanner produced images. All DICOM images of three routine DW-EPI acquisitions were also transferred to workstation for later data analysis.
Data Analysis: ADC maps were generated by using a pixel-by-pixel computation for all datasets using Matlab. In each dataset, a representative slice was selected for the ADC measurement, and three circular regions-of-interest (ROIs) with 100 pixels in each were manually placed in the peripheral liver parenchyma to avoid partial volume effect of blood vessels and bile ducts. The measured ADC values of two repeated scans for each type of sequences were averaged together for the group comparison using student t-test. Repeatability of ADC measurements of all sequences were determined according to the methods of Bland and Altman, and quantitatively assessed using the inter-class correlation coefficient (ICC) and coefficient of variation (CV).
RESULTS
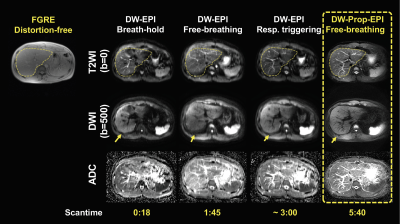
Figure 2 compares the individual blade images acquired using DW-Propeller-EPI with and without VC diffusion gradient for the same subject under free-breathing condition. Figure 3 shows the representative liver diffusion images and corresponding ADC maps generated from three routine DW-EPI methods and free-breathing DW-Propeller-EPI. Figures 4 and 5 show the comparison of measured ADC values and repeatability of ADC measurement between different sequences, respectively.DISCUSSION
The discrepancy of ADC measurement can substantially cause difficulty in cross-sectional or longitudinal liver DWI. Therefore, the knowledge of repeatability (inter-scan) or reproducibility (inter-scanner) of selected liver DWI sequence is critical to ensure correct interpretation in measured ADC value. In our preliminary evaluation, the ADC measurement using free-breathing liver DW-Propeller-EPI is better repeatable than other three routine liver DW-EPI methods (Fig.5). The improved repeatability is possibly owing to the reduced susceptibility to respiration motion for DW-Propeller-EPI by using VC diffusion gradient (Fig.2). In addition, the significant improvement of image quality in DW-Propeller-EPI, such as high geometric accuracy and less image blurring (Fig.3), may also contribute to produce a more accurate ADC map (lower row in Fig.3), thereby improving the repeatability. In this study, the respiratory-triggered DW-EPI is also found to be less repeatable than breath-hold and free-breathing DW-EPI that perfectly agrees with the findings in a previous study13. However, the respiratory-triggered DW-EPI is still preferred for routine liver DWI because the free-breathing acquisition may lead to additional image blurring associated with respiratory motion (Fig.3). The DW-Propeller-EPI can enable free-breathing liver DWI without suffering from image blurring and degraded image quality, and therefore better suits the subjects with irregular respiration. Besides, the ADC measurement using free-breathing DW-Propeller-EPI is comparable to respiratory-triggered DW-EPI, thus allowing direct adoption of existing applications for liver DWI (Fig.4). One disadvantage of DW-Propeller-EPI is lengthened scantime due to multi-shot acquisition (5:40 in this study), but nevertheless the improved image quality is worth the lengthened scantime. In conclusion, free-breathing liver DW-Propeller-EPI can significantly improve the image quality and repeatability of ADC measurement, and therefore may be a superior alternative to DW-EPI for liver DWI applications.Acknowledgements
The work was in part supported by grants from Hong Kong Research Grant Council (GRFs HKU17138616, HKU17121517 and HKU17106820) and Hong Kong Innovation and Technology Commission (ITS/403/18).References
1. Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 2008;246(3):812-822.
2. Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, Motoori K, Ueda T. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology 2006;239(1):122-130.
3. Amano Y, Kumazaki T, Ishihara M. Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol 1998;39(4):440-442.
4. Lewin M, Poujol-Robert A, Boelle PY, Wendum D, Lasnier E, Viallon M, Guechot J, Hoeffel C, Arrive L, Tubiana JM, Poupon R. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology 2007;46(3):658-665.
5. Girometti R, Furlan A, Esposito G, Bazzocchi M, Como G, Soldano F, Isola M, Toniutto P, Zuiani C. Relevance of b-values in evaluating liver fibrosis: a study in healthy and cirrhotic subjects using two single-shot spin-echo echo-planar diffusion-weighted sequences. J Magn Reson Imaging 2008;28(2):411-419.
6. Ni P, Lin Y, Zhong Q, Chen Z, Sandrasegaran K, Lin C. Techncial advancements and protocol optimization of diffusion-weighted imaging (DWI) in liver. Abdominal Radiology 2016;41:189-202.
7. Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 2006;24(3):478-488.
8. Bammer R, Keeling SL, Augustin M, Pruessmann KP, Wolf R, Stollberger R, Hartung HP, Fazekas F. Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE). Magn Reson Med 2001;46(3):548-554.
9. Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging 2012;36(1):55-72.
10. Lewis S, Dyvorne H, Cui Y, Taouli B. Diffusion-weighted imaging of the liver: techniques and applications. Magn Reson Imaging Clin N Am 2014;22(3):373-395.
11. Ozaki M, Inoue Y, Miyati T, Hata H, Mizukami S, Komi S, Matsunaga K, Woodhams R. Motion artifact reduction of diffusion-weighted MRI of the liver: use of velocity-compensated diffusion gradients combined with tetrahedral gradients. J Magn Reson Imaging 2013;37(1):172-178.
12. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology 2010;254(1):47-66.
13. Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging 2008;28(5):1141-1148.
14. Chang HC, Chen NK, Juan CJ, Chuang TC, Ko CW, Chung HW. Free breathing Liver DWI using PROPELLER-DW-EPI with inherent reductions of geometric distortion and motion at 1.5T. ISMRM, 20th Annual Meeting, Melbourne, Australia, May 2012. (Proc Intl Soc Mag Reson Med 2011;p.261)
Figures