0304
Correcting vs resolving respiratory motion in accelerated free-running whole-heart radial flow MRI using focused navigation (fNAV)1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Department of Biomedical Engineering, Northwestern University, Chicago, IL, United States, 4Advanced clinical imaging technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 6Woman-Mother-Child Department, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 7Service of Cardiology, Centre de resonance magnétique cardiaque (CRMC), Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
In this work, a free-running radial whole-heart flow sequence was acquired in five congenital heart disease patients and images were reconstructed using a) a previously developed 5D flow framework for respiratory and cardiac resolved images, and b) a novel framework for respiratory motion corrected and cardiac resolved 4D flow (fNAV). Image and flow differences were measured across a range of acceleration factors. We showed that the free-running acquisition, which is already undersampled, can be even further accelerated with less signal degradation if it is reconstructed with fNAV 4D flow, compared to using 5D flow.
Introduction
4D flow MRI is typically acquired over several minutes during free-breathing and, therefore, requires respiratory motion compensation. This is typically achieved using respiratory navigators that limit data acquisition to a predetermined respiratory level, resulting in decreased scanning efficiency1,2. Alternatively, data acquired throughout the entire respiratory cycle may be used for image reconstruction by either correcting respiratory motion3,4, or by binning the data into unique phases to resolve respiratory motion, allowing for more predictable scanning times5–7. The increased scanning efficiency derived from correcting or resolving respiratory motion may be used for improved resolution, volumetric coverage, or to further accelerate the already undersampled acquisitions8,9. In this work, we present a novel approach for respiratory motion correction in free-running radial whole-heart flow MRI based on focused navigation (fNAV)10. We compared respiratory corrected fNAV 4D flow to respiratory resolved 5D flow6 reconstructions of the same datasets across a range of undersampling factors, and tested the hypothesis that fNAV 4D flow enables higher acceleration factors than the ones already achieved in free-running flow, as opposed to 5D flow.Methods
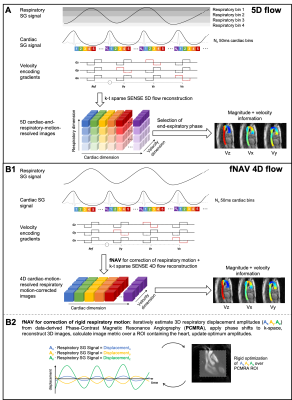
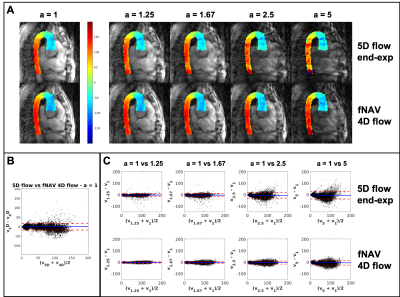
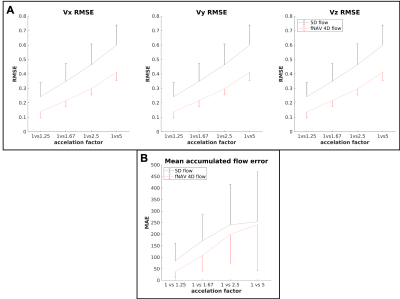
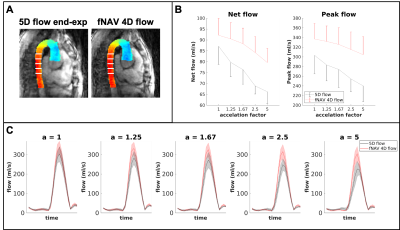
Five congenital heart disease patients (18-29 years, 3 Female) were scanned after ferumoxytol contrast administration (dose 2mg/kg) on a 1.5T clinical MR system (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany) using a prototype free-running radial whole-heart flow sequence6,11. The study was approved by the local ethics committee and all patients provided written informed consent compliant with our institutional guidelines. Scan parameters (Scan time=8:08-8:14min, field of view=(220 mm)3-(260 mm)3, spatial resolution=(2.3 mm)3-(2.7 mm)3, temporal resolution=40ms, Venc=200cm/s) were adjusted for each patient. Two reconstruction methods were used: a) extraction of respiratory and cardiac self-gating signals6,12 for data sorting into a motion-resolved 5-dimensional flow array and k-t-sparse SENSE reconstruction6,11(Fig.1A). b) cardiac self-gating signal extraction for data sorting, and respiratory signal extraction to use as input for the fNAV auto-focusing algorithm(Fig.1B)10. fNAV 4D flow reconstructions were similar to 5D flow6, but with no respiratory regularization. 5D flow images at end-expiration were manually selected. To study the acceleration effect, we reconstructed fNAV 4D flow and 5D flow images using [100,80,60,40,20]% of the acquired data (retrospective acceleration factors a=[1,1.25,1.67,2.5,5], relative to originally undersampled datasets6). Qualitative comparison of all reconstructions was performed by calculating the maximum intensity projection (MIP) of the dominant velocity direction (z) in a sagittal reformat. Then, using a 3D segment of the aorta, a voxel-wise Bland-Altman plot was computed to estimate the intra-voxel variability across the different acceleration factors in comparison to (a=1). The root mean square error(RMSE) of all subjects was calculated between every acceleration factor and (a=1), across all voxels and cardiac phases. Five 2D-orthogonal planes were segmented in the descending aorta for each reconstructed image, and the flow curves, net flow, and peak flow measurements were calculated. The mean accumulated flow error13 was computed for each 2D plane as the difference between the flow curves of each acceleration factor with respect to (a=1).Results
MIP images of the aorta showed good visual agreement between fNAV 4D flow and 5D flow (Fig.2A) for reconstructions using all acquired data (a=1). For increasing acceleration factors, degradation of 5D flow reconstructions is noticeable while fNAV 4D flow remains relatively consistent. These qualitative results are corroborated by the Bland-Altman plots of the velocity vector for increasing acceleration factors (Fig.2B-C), showing an overall larger standard deviation within the 5D flow datasets. RMSE (Fig.3A) and mean accumulated flow error (Fig.3B) increased with acceleration factor for both fNAV 4D flow and 5D flow as expected, but the error was higher overall in magnitude and variability for the 5D flow images. Higher net flow and peak flow measurements (Fig.4) were reported in fNAV 4D flow images, relative to 5D flow, while also being more robust to increasing acceleration factors.Discussion
fNAV 4D flow provided comparable, albeit slightly increased net and peak flow measurements, relative to 5D flow, and showed less degradation in overall reconstruction and flow errors for the tested acceleration factors, confirming therefore our original hypothesis. Our results, albeit with a small sample size, suggest that acquisitions reconstructed with fNAV 4D flow could be prospectively accelerated by a larger factor than when using 5D flow, and without significant degradation of image quality or flow measurements. For instance, using 60% of the original data for reconstruction with fNAV 4D flow (a=1.67) would grant similar flow measurements from the original data size, and would provide lower signal corruption and flow variability than a 5D flow reconstruction using only 80% of the data (a=1.25).Intra-voxel variability was reported between the two reconstructions, so further comparisons to established Cartesian 2D or 4D flow are needed to determine which of these methods better represents ground truth. Nevertheless, 5D flow has previously reported underestimated flow measurements relative to Cartesian 4D flow, possibly due to physiological variation or overregularization from the reconstruction algorithm. fNAV 4D flow may, thus, help elucidate whether increasing the amount of data per cardiac bin improves the agreement between the proposed radial and established Cartesian flow measurements.Conclusion
Our results suggest the robustness of fNAV 4D flow across the tested acceleration factors in comparison to 5D flow, may enable faster scan times, improved resolution, or better agreement with the established Cartesian flow measurements.Acknowledgements
Grant support by the Swiss National Science Foundation Grant/Award 173129 and the National Institutes of Health Grants R01HL115828 and F30HL137279.References
1. Markl, Michael, Alex Frydrychowicz, MD, Sebastian Kozerke MH, Wieben O. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015-1036. doi:10.1007/978-3-319-65924-4_9
2. Dyverfeldt P, Bissell M, Barker AJ, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson. 2015;17(1):1-19. doi:10.1186/s12968-015-0174-5
3. Cheng JY, Hanneman K, Zhang T, et al. Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease. J Magn Reson Imaging. 2016;43(6):1355-1368. doi:10.1002/jmri.25106
4. Kolbitsch C, Vasquez CP, Bastkowski R, Weiss K, Maintz D. Respiratory motion corrected 4D flow using golden radial phase encoding. Magn Reson Med. 2019;(June):00:1-10. doi:10.1002/mrm.27918
5. Walheim J, Dillinger H, Kozerke S. Multipoint 5D flow cardiovascular magnetic resonance - Accelerated cardiac- and respiratory-motion resolved mapping of mean and turbulent velocities. J Cardiovasc Magn Reson. 2019;21(1):1-13. doi:10.1186/s12968-019-0549-0
6. Ma LE, Yerly J, Piccini D, et al. 5D Flow MRI : A Fully Self-gated , Free-running Framework for Cardiac and Respiratory Motion – resolved 3D Hemodynamics. Radiol Cardiothorac Imaging. 2020;2(6)(6).
7. Bastkowski R, Bindermann R, Brockmeier K, Weiss K. Respiration Dependency of Caval Blood Flow in Patients with Fontan Circulation : Quantification Using 5D Flow MRI. Radiol - Cardiothorac Imaging. 2019;1(4).
8. Ma LE, Markl M, Chow K, et al. Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k-space reordering, and inline reconstruction. Magn Reson Med. 2019;81(6):3675-3690. doi:10.1002/mrm.27684
9. Giese D, Schaeffter T, Kozerke S. Highly undersampled phase-contrast flow measurements using compartment-based k-t principal component analysis. Magn Reson Med. 2013;69(2):434-443. doi:10.1002/mrm.24273
10. Roy CW, Heerfordt J, Piccini D, et al. Motion Compensated Whole-Heart Coronary Magnetic Resonance Angiography using Focused Navigation (fNAV). 2020:0-2. http://arxiv.org/abs/2010.14206.
11. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med. 2019;(June):1-15. doi:10.1002/mrm.27898
12. Falcão M, Ma L, Di Sopra L, et al. 5D Flow MRI using Pilot Tone for Cardiac and Respiratory Self-Gating. Proc 23rd Annu SCMR Sci Sess. 2020:1583-1586.
13. Giese D, Wong J, Greil GF, Buehrer M, Schaeffter T, Kozerke S. Towards highly accelerated Cartesian time-resolved 3D flow cardiovascular magnetic resonance in the clinical setting. J Cardiovasc Magn Reson. 2014;16(42):1-8. doi:10.1186/1532-429X-16-42
Figures