0301
Does the internal carotid artery attenuate blood-flow pulsatility in small vessel disease? A 7T 4D-flow MRI study.1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology, UMC Utrecht, Utrecht, Netherlands
Synopsis
We studied blood-flow pulsatility and arterial distensibility along the internal carotid artery (ICA) in cerebral small vessel disease (CSVD) patients and healthy controls using 7Tesla MRI. 4D-flow measurements (0.8 mm isotropic resolution), were analyzed in 17 patients with lacunar infarcts or deep intracerebral hemorrhage (CSVD) and 17 age and sex matched healthy controls. Pulsatility was significantly higher and arterial distensibility significantly lower in CSVD patients compared to controls. Velocity pulsatility was attenuated between the extracranial ICA and the circle of Willis in controls, but increased in CSVD. Higher calcification in CSVD patients correlated with reduced distensibility and increased velocity pulsatility.
Introduction
Increased cerebral blood flow pulsatility is associated with stroke, cognitive impairment and characteristic lesions of cerebral small vessel disease (CSVD), including lacunar strokes, microbleeds and white matter lesions (1,2). Maintaining low cerebral flow pulsatility depends on effective attenuation of the cardiac pulse pressure along the carotid artery. Recently, it has been suggested that the internal carotid artery (ICA) siphon functions as an attenuator for intracranial pulsatility (3,4). Other research has shown that the siphon is a common location for non-atherosclerotic calcifications (5,6). This intracranial internal carotid artery calcification (iICAC) is mainly located in the elastic lamina, and associated with increased arterial stiffness,(7) which might directly contribute to increased pulsatility by increasing stiffness of the arterial walls. iICAC is associated with cognitive impairment and characteristic lesions of CSVD (8). Increased pulsatility in cerebral perforating arteries in patients with CSVD has already been shown (9), but attenuation of the pulsatility along the ICA in relation with its distensibility and the presence of iICAC has not yet been studied in CSVD patients. This study aims to compare the blood-flow pulsatility and arterial distensibility pattern along the ICA between the CSVD group and healthy controls using 7Tesla MRI. Additionally, this study explores the influence of iICAC on pulsatility and distensibility along the ICA (C1 to C7) (10) towards the circle of Willis in patients with CSVD.Methods
A previous study described velocity pulsatility in the cerebral perforating arteries in patients with lacunar infarcts or deep intracerebral hemorrhage (CSVD group) and age and sex matched controls (9). From this study, we selected participants with complete 4D-flow datasets covering the internal carotid arteries and circle of Willis. Time-resolved measurements of blood velocities and volumetric flow rates over the whole ICA trajectory were acquired with four-dimensional phase-contrast MRI (4D PC-MRI) using 7Tesla MRI (Philips Healthcare). 4D phase-contrast MRI parameters: acquired resolution 0.8x0.8x0.8mm3, velocity encoding sensitivity (Venc) 100cm/s (separate acquisitions for Feet-Head, Right-Left and Anterior-Posterior velocity encodings), angulated coronal field of view 250 (Feet-Head) x 190 (Right-Left) x 20 (Anterior-Posterior) mm, flip angle 15°, acquired temporal resolution 65 ms, acquisition duration 3x4:55 min:sec for a heart rate of 60 bpm. Datasets were analyzed using CAAS software (Pie Medical Imaging, Maastricht, The Netherlands) (Figure 1). The blood-flow velocity pulsatility index (vPI = (Velocitymax-Velocitymin)/Velocitymean) and arterial distensibility (ΔD/(ΔP x D)*100) (11) were calculated for all ICA segments (C1-C7), where D indicates the diameter of the ROI and ΔP is systolic pressure – diastolic pressure obtained from blood pressure measurements, measured on the day of study participation (last measurement out of 3). In CSVD patients, presence and volume of iICAC were determined on CT scans, where the iICAC volume was determined between C3 and C7. CT scans were not available in controls. The influence of iICAC, age, sex, hypertension and hyperlipidemia on vPI and distensibility was tested using mixed-model analysis (SPSS). For the relation between iICAC volume and vPI or distensibility, average values for vPI and distensibility over C3-C7 were used.Results / Discussion
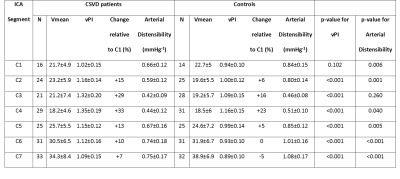
4D flow datasets were available in 17 CSVD patients and 17 age and sex matched, healthy controls (Table 1). vPI was significantly higher (except for C1) and distensibility was significantly lower (except for C3) in CSVD patients. In both groups vPI and distensibility variation along the carotid artery were similar (Table 2, Figure 2). vPI and distensibility were negatively correlated in the whole group R[95% confidence interval] = -0.68 [-0.94 ; -0.43], so higher distensibility results in lower velocity pulsatility. vPI was attenuated between C1 and C7 in controls (vPIC7-vPIC1=-4.6±3.6%; average of both ICAs), but increased in CSVD patients (vPIC7-vPIC1=+6.5±3.1%). The changes in vPI between C1 and C7 were statistically significant different from zero for both groups (p=0.002). vPI was positively associated with hypertension in both groups (CSVD p<0.001, healthy controls p=0.026). After correcting for age, sex, hypertension, hyperlipidemia and stenosis >50% using the mixed model analysis the CSVD group still had a higher vPI (beta [95% CI] 0.18 [0.15; 0.21]) and a lower distensibility (beta [95% CI] 0.26 [0.22; 0.29]) compared to controls. Also after including the systolic and diastolic blood pressure as continuous variable the CSVD group still had higher vPI (beta [95% CI] 0.19 [0.16; 0.22]) and lower distensibility (beta [95% CI] 0.24 [0.20; 0.27]). The right and left ICA volume of calcification correlated with average distensibility in both right (r= -0.411, p=0.013) and left ICA (r= -0.407, p=0.018) and vPI in both right (r= 0.645, p=0.004) and left ICA (r= 0.633, p=0.001). The change in vPI between C1 and C7 correlated with calcification volume (p=0.02) and age (p=0.03) (i.e. higher vPI at C7 with higher calcification volume or age).Conclusion
These results suggest that decreased distensibility and subsequently reduced pulsatility attenuation along the ICA contribute to CSVD. Accordingly, previously observed increased pulsatility in the microvasculature (9) of these patients might be associated with reduced distensibility of the carotids and reduced pulsatility damping along the carotid siphon.Acknowledgements
We thank the study participants and magnetic resonance technicians for their support and participation. We acknowledge the support of The Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2015‐008 ERASE), Dutch Federation of University Medical Centers, The Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreements n°337333 and n° 841865.References
1. Shi Y, Thrippleton MJ, Blair GW, et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab. 2020;40(1):85-99.
2. Birnefeld J, Wåhlin A, Eklund A, Malm J. Cerebral arterial pulsatility is associated with features of small vessel disease in patients with acute stroke and TIA: a 4D flow MRI study. J Neurol. 2020;267(3):721-730.
3. Schubert T, Santini F, Stalder AF, et al. Dampening of blood-flow pulsatility along the carotid siphon: Does form follow function? Am J Neuroradiol. 2011;32(6):1107-1112.
4. van Tuijl RJ, Ruigrok YM, Velthuis BK, van der Schaaf IC, Rinkel GJE, Zwanenburg JJM. Velocity Pulsatility and Arterial Distensibility Along the Internal Carotid Artery. J Am Heart Assoc. 2020;9(16):e016883.
5. Vos A, Van Hecke W, Spliet WGM, et al. Predominance of nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke. 2016;47(1):221-223.
6. Fisher CM, Gore I, Okabe N, White PD. Calcification of the carotid siphon. Circulation. 1965;32(4):538-548.
7. Odink AE, Mattace-Raso FUS, van der Lugt A, et al. The association of arterial stiffness and arterial calcification: The Rotterdam Study. J Hum Hypertens. 2008;22(3):205-207.
8. Wu XH, Chen XY, Wang LJ, Wong KS. Intracranial artery calcification and its clinical significance. J Clin Neurol. 2016;12(3):253-261.
9. Geurts LJ, Zwanenburg JJM, Klijn CJM, Luijten PR, Biessels GJ. Higher Pulsatility in Cerebral Perforating Arteries in Patients with Small Vessel Disease Related Stroke, a 7T MRI Study. Stroke. 2019;50(1):62-68.
10. Bouthillier A, Van Loveren HR, Keller JT. Segments of the internal carotid artery: A new classification. Neurosurgery. 1996;38(3):425-433.
11. O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426-444.
Figures
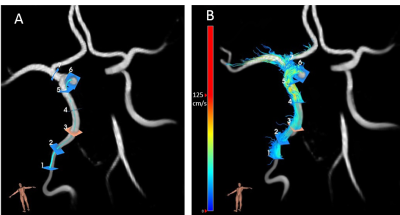
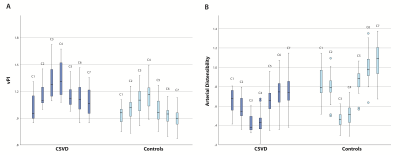
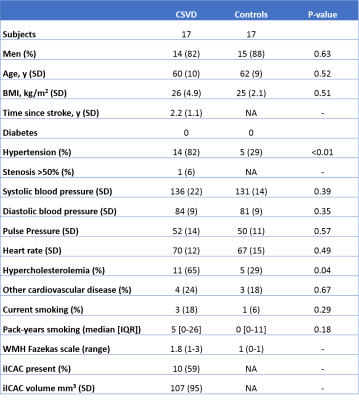
Table 1: Baseline characteristics.
BMI: Body Mass Index; CSVD: Cerebral Small Vessel Disease; IQR: Interquartile range; NA: not available; SD: standard deviation. WMH: White Matter Hyperintensities; iICAC: Intracranial internal carotid artery calcification.