0300
Ex-vivo whole human brain high b-value diffusion MRI at 550 micron with a 3T Connectom scanner1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Masachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Q Bio Inc, San Carlos, CA, United States, 4Institute of Medical Physics and Radiation Protection, Mittelhessen University of Applied Sciences, Giessen, Germany
Synopsis
We demonstrate high quality, high b-value diffusion MRI (up to 10 000 s / mm2) in an ex-vivo human brain at ultra-high spatial resolution (550 micrometer). The high signal quality allows us to obtain high diffusion contrast and delineate fine structures that cannot be resolved within in vivo acquisitions. We present DTI and DKI results of the whole brain, show diffusivity on the primary somatosensory, auditory, and visual cortex areas as well as illustrate tractography of the hippocampus and thalamus structures, revealing internal connectivity at a high level of detail.
Introduction
Ex vivo diffusion MRI is an indispensable research tool for probing and validating the multiscale nature of human brain circuitry1-4. Here, we demonstrate that ex vivo diffusion MRI at high gradient strengths coupled with the use of a custom-designed receiver coil enables the acquisition of comprehensive ex vivo diffusion MRI data in a whole human brain specimen at high b-values up to b=80,000 s/mm2 and ultra-high spatial resolution (down to 550 um isotropic voxel size). We illustrate a variety of diffusion analyses in brain regions that present challenges to in-vivo diffusion MRI and highlight the capability of multi-b-value acquisitions in ex vivo specimens to validate measures of tissue microstructure by diffusion MRI.Method
Data acquisition, reconstruction and processing:A whole fixed human brain from a male who died of non-neurological causes was scanned on a dedicated high-gradient 3T MRI scanner (MAGNETOM Connectom, Siemens Healthineers) equipped with maximum gradient strength of 300 mT/m. The excised brain was placed in fixative (10% formaldehyde) for 90 days and transferred to paraformaldehyde-lysine-periodate solution for long-term storage. A custom-made 48-channel phased array coil for ex vivo whole brain imaging was used for signal reception7. Data were acquired at 550 um isotropic resolution using a 3D segmented-EPI diffusion-weighted spin echo sequence.3 The multi-shell diffusion MRI protocol consisted of 16 diffusion-weighted volumes at b = 4,000 s/mm2 and 36 diffusion-weighted volumes at b=10,000 s/mm2, with directions equally distributed over the sphere. Other relevant image acquisition parameters were: matrix size=282x192x288, 13 shots, 15 lines/shot, TE/TR=68/500 ms. Total acquisition time was 9.8h for the b=4,000 s/mm2 scan and 19.2h for the b=10,000 s/mm2 scan (31.2 min/volume). A separate multi-shell acquisition was performed at 800 um isotropic resolution using b-values ranging from 2,000 to 80,000 s/mm2. Real-valued DWIs were obtained8 and eddy current distortions were corrected.9
Data analysis:
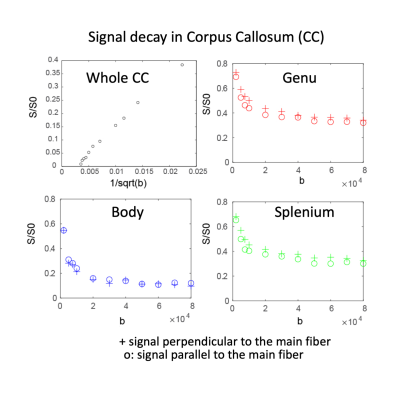
Diffusion tensor analysis and mean kurtosis was estimated with dtifit in FSL. In addition, fODFs were fitted using MSMT-CSD11,12 in MRtrix310. Probabilistic tractography was performed to obtain 100K streamlines with whole-brain seeding. fODF reconstruction and tractography were repeated after downsampling the data by a factor of 3 with nearest-neighbor interpolation. For the multi-b-value data, the spherical average of the diffusion-weighted signal was calculated. Generalized q-sampling imaging was used to determine the principal fiber direction, from which the normalized signals parallel and perpendicular to this orientation were extracted.15 Regions of interest for whole midline corpus callosum, as well as subportions (genu, body and splenium) were delineated by an expert neuroanatomist.
Results
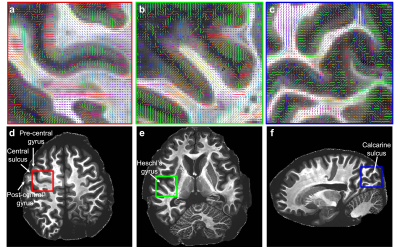
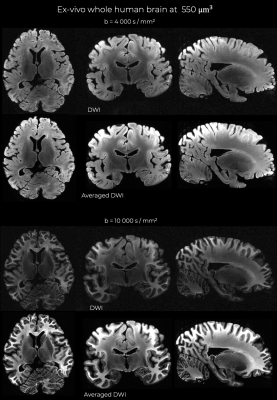
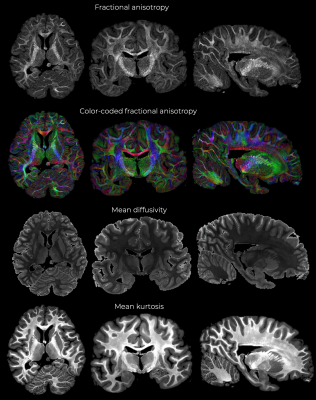
Figure 1 shows representative individual DWIs and the spherically averaged DWIs at 550 um isotropic resolution for b=4000 and 10,000 s/mm2. Figure 2 shows maps of FA, MD and mean kurtosis derived from this data. The ultra-high spatial resolution enables imaging of the claustrum, a structure that typically appears as a faint line on in vivo scans, and to follow fibers as they branch at the level of the claustrum to enter the external and extreme capsules (Fig. 3I). Fig.3II shows coronal and sagittal views of the hippocampus: the substructures of the hippocampus are distinguishable in the 0-th order spherical harmonic map (Fig.3II.a1-b1), and further resolved with the aid of probabilistic tractography, which shows its internal connectional anatomy (Fig.3II.a2-b2). This level of detail is lost when the data are downsampled to a more conventional DWI resolution (Fig.3II.a3-3). Fig. 4 demonstrates that principal diffusion orientations tangential to the cortical surface are detected in primary somatosensory, auditory and visual cortex areas. Fig. 5 shows that the spherically averaged signal plotted as a function of 1/sqrt(b) yields a negative intercept at b=0 after subtracting a fraction of stationary water estimated from the parallel signal. The perpendicular signal decays in the genu, body and splenium of the corpus callosum are consistent with the overall trends in signal attenuation measured in vivo, while the parallel signal demonstrates different behavior from the Gaussian, mono-exponential signal decays observed in vivo.Discussion
Prior studies using human brain samples scanned in small-bore scanners have shown that spatial resolution may have a bigger impact than angular resolution on the accuracy of dMRI-derived fiber orientation estimates13. Here, we acquired whole-brain data at a resolution as high as 550 um. These data could be used to obtain high-quality models of white-matter anatomy, which can then be used to train algorithms for improved reconstruction of this anatomy from lower-quality data14. Data obtained at slightly lower resolution (800 um) with multiple b-values demonstrate similar scaling of the spherically averaged signal as a function of 1/sqrt(b), implying that a finite axon diameter may be estimated from this data,16 opening up the possibility of performing multi-scale validation of in vivo diffusion MRI measures using ex vivo diffusion MRI as a key bridging technology.Conclusion
Whole-brain ex-vivo diffusion MRI at high b-values and ultra-high spatial resolution will open the door to richer analyses of human brain anatomy and circuitry, offering the capability to map mesoscale connectional anatomy and microstructure with unprecedented data quality. In addition to furthering our understanding of human neuroanatomy, these data will be invaluable for validating biophysical models and methods for mapping connectional anatomy from in vivo diffusion MRI.Acknowledgements
This work was supported by the NIH (grants P41-EB030006, U01-EB026996, R01-EB021265, K23-NS096056).References
- Roebroeck, A., Miller, K. L., & Aggarwal, M. (2019). Ex vivo diffusion MRI of the human brain: Technical challenges and recent advances. NMR in Biomedicine, 32(4), e3941.
- McNab, J. A., Jbabdi, S., Deoni, S. C., Douaud, G., Behrens, T. E., & Miller, K. L. (2009). High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession. Neuroimage, 46(3), 775-785.
- Miller, K. L., Stagg, C. J., Douaud, G., Jbabdi, S., Smith, S. M., Behrens, T. E., ... & Jenkinson, N. (2011). Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage, 57(1), 167-181.
- Fritz, F. J., Tse, D. H. Y., & Sengupta, S. (2017). High resolution diffusion MRI and tractography of post mortem human brains using kT‐dSTEAM at 9.4 T. Organization for Human Brain Mapping.
- McNab, J. A., Polimeni, J. R., Wang, R., Augustinack, J. C., Fujimoto, K., Stevens, A., ... & Wald, L. L. (2013). Surface-based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex. Neuroimage, 69, 87-100.
- Leuze, C. W., Anwander, A., Bazin, P. L., Dhital, B., Stüber, C., Reimann, K., ... & Turner, R. (2014). Layer-specific intracortical connectivity revealed with diffusion MRI. Cerebral Cortex, 24(2), 328-339.
- Scholz, A, et al., “A 48-channel ex vivo Brain Array Coil for Diffusion-Weighted MRI at 3T”, Proc. Intl. Soc. Mag. Reson. Med. 27 (2019), 1494
- Eichner, C., Cauley, S. F., Cohen-Adad, J., Möller, H. E., Turner, R., Setsompop, K., & Wald, L. L. (2015). Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. NeuroImage, 122, 373-384.
- Andersson, J. L., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, 1063-1078.
- Jeurissen, B., Tournier, J. D., Dhollander, T., Connelly, A., & Sijbers, J. (2014). Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage, 103, 411-426.
- T. Dhollander, R. Mito, D. Raffelt, and A. Connelly. Improved white matter response function estimation for 3-tissue constrained ... Proc. Intl. Soc. Mag. Reson. Med. 27 (2019),555
- Tournier, J. D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., ... & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137.
- Jones R, Grisot G, Augustinack J, Magnain C, Boas DA, Fischl B, Wang H, Yendiki A. Insight into the fundamental trade-offs of diffusion MRI from polarization-sensitive optical coherence tomography in ex vivo human brain. Neuroimage. 2020 Jul 1;214:116704.
- C. Maffei, A. Yendiki. Using HCP data to improve diffusion tractography in routine-quality data: Application to the virtual dissection of the SLF system, Proc. Intl. Soc. Mag. Res. Med., 2019.
- Huang, Susie Y., et al. "High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain." Brain Structure and Function 225.4 (2020): 1277-1291.
- Veraart J, Nunes D, Rudrapatna U, Fieremans E, Jones DK, Novikov DS, Shemesh N. Noninvasive quantification of axon radii using diffusion MRI. Elife. 2020 Feb 12;9:e49855.
Figures
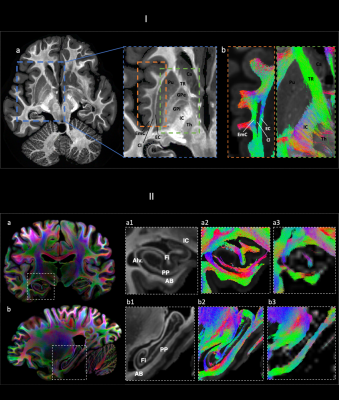
I. a) Mean kurtosis b) tractography showing branching between EC and EmC (left) and thalamic connectivity (right). Ca: caudate. Cl: claustrum. EC: external capsule. Emc: extreme capsule. IC: internal capsule. GPi/GPe: internal/external globus pallidus. Th: thalamus. Pu: putamen. TR: thalamic radiation.
II a-b) CSD a-b1,2,3: in a-b. 1) CSD-based maps (0-th order SH) showing main hippocampal formation structures. 2) tractography showing internal hippocampal connectivity. 3) lower spatial resolution .