0298
Tensor-valued Diffusion MRI Shows Elevated Microscopic Anisotropy and Tissue Heterogeneity in White and Grey Matter of Acute Ischemic Stroke1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Clinical Sciences Lund, Lund University, Lund, Sweden, 3Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States, 4Neurology, University of Alberta, Edmonton, AB, Canada, 5Radiology and Diagnostic Imaging, University of Alberta, Edmonton, AB, Canada
Synopsis
Novel diffusion encoding modalities, such as tensor-valued encoding, can disentangle the effects of intra-voxel orientation dispersion and diffusion anisotropy, thereby resolving the fiber density from tissue heterogeneity. A rapid 2.5-minute protocol for tensor-valued diffusion MRI was applied for the first time to acute stroke. Microscopic anisotropy (µFA and MKA) and tissue heterogeneity (MKI) were higher in lesions of white and grey matter, in contrast to reduced DTI-derived fractional anisotropy at the voxel level. Elevated microscopic anisotropy in acute stroke may reflect increased trapped water in swollen axons, a measure independent of tract orientation dispersion.
Introduction
Diffusion-weighted imaging (DWI) is used daily to diagnose stroke, however there are uncertainties in the microstructural changes associated with the lower mean diffusivity (MD) that identify acute ischemic brain lesions. Novel multidimensional (b-tensor) diffusion encoding methods may provide insight by disentangling effects of microscopic heterogeneity and tract orientation dispersion, yielding measurements of microscopic anisotropy (e.g., micro-fractional anisotropy – μFA and anisotropic kurtosis – MKA) and isotropic diffusional variance within a voxel (isotropic kurtosis – MKI)1-5. Clinical applications of such b-tensor methods have been limited to brain tumors4-6, white matter lesions with aging7, cortical malformations in epilepsy8, and multiple sclerosis9, where measurements have been linked to cell eccentricity and variable cell density. We aim to evaluate microscopic anisotropy and isotropic diffusional variance in ischemic white (WM) and grey matter (GM) using b-tensor encoding in acute human stroke and to compare these metrics to standard ‘macroscopic’ FA derived from diffusion tensor imaging (DTI)10.Methods
Both linear (LTE) and spherical (STE) b-tensor encoding11, 12 were performed with a prototype single shot spin-echo EPI diffusion sequence13 on a 3T Siemens Prisma using a 64 channel head coil with: 15 axial slices, 3 mm thick with no gap centered on the lesion, 2x2 mm2 in-plane resolution, GRAPPA R=2, PPF6/8, TR 2000 ms, TE 91 ms, LTE with 6 b=100, 12 b=1000, and 22 b=2000 s/mm2 and STE with b=100, 500, 1000, 1500, 2000 s/mm2 in 6 directions each for a total scan time of 2:32 min (kept short for the acute stroke patients). The 21 stroke patient volunteers were: 66 ± 17 (28-95) years old, 16 males/5 females, NIH stroke scale score of 5±5 (0-21), scanned 22±13 (3-57) hours after stroke onset, and had lesion volumes of 12 ± 20 (0.2-80) cm3. The q-space trajectory imaging signal representation was smoothed and fit to LTE and STE data using open source code3, 14 to generate voxel-by-voxel maps of MD, FA, μFA, as well as total, anisotropic and isotropic diffusional variance (MKT, MKA, MKI). Regions-of-interest over multiple slices were placed manually in WM (n=21) and GM (n=14) (delineated on FA and µFA maps) within the acute lesion (identified by low MD) or the corresponding contralateral hemisphere for comparison (Mann-Whitney U-test) of the diffusion metrics listed above. Linear correlation between FA and μFA was assessed for WM and GM separately. Additionally, the anisotropy difference between lesions and contralateral tissue per patient was compared for ΔFA vs. ΔμFA.Results
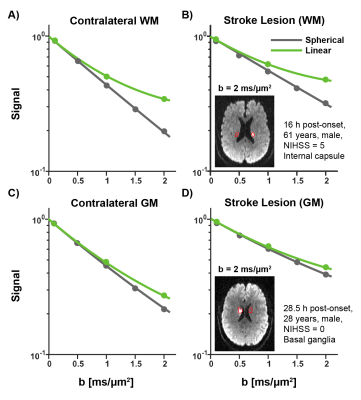
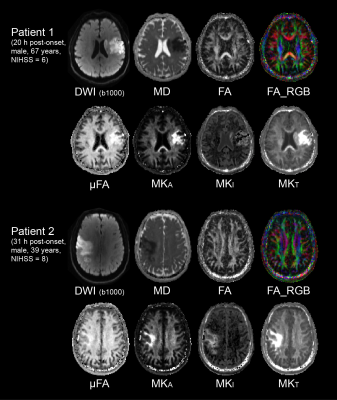
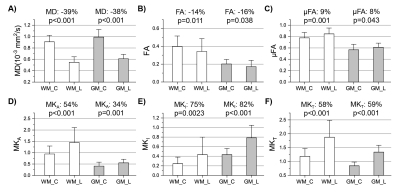
Signal is less attenuated with b-value in the lesions compared to contralateral tissue, as expected for reduced diffusivity (Figure 1). Accounting for change in MD, the difference between STE and LTE reflects greater tissue anisotropy in lesions (and in WM versus GM), while the curvature of STE itself reflects isotropic diffusional variance. The fitting of the LTE and STE data yields the tensor (FA, MD) and b-tensor (μFA and MKA/I/T) maps as shown in two example acute stroke patients (Figure 2). The ischemic region with typical hyper-intensity on DWI with concurrent lower MD demonstrates elevated µFA, MKI, and MKA mainly in the lesion white matter, but there is no evident change of FA.In all 21 patients, lesion MD was reduced by 39% in WM and 38% in GM (Figure 3A) with greater proportional increases of MKT by 58% and 59%, respectively (Figure 3F). Interestingly, ischemic WM showed a 14% lower FA than contralateral WM (Figure 3B) whereas MKA was 54% higher (Figure 3D) and μFA was 9% higher (Figure 3C). MKI showed the greatest proportional increase (75%) in ischemic WM (Figure 3E). Similar changes in all metrics were present in ischemic GM.
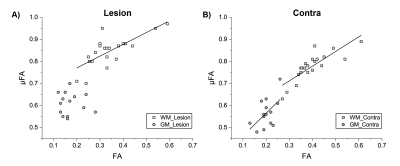
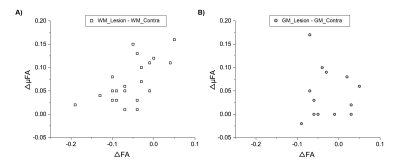
A strong linear correlation is observed between FA and µFA in lesion WM (r=0.74, p=0.00013) and contralateral WM (r=0.83, p=0.0000036), along with a moderate correlation in contralateral GM (r=0.54, p=0.04) (Figure 4). However, all 21 WM lesions showed ΔμFA greater than zero with 10 of these showing FA reductions of > -0.05 (Figure 5). The 7 WM lesions with the highest ΔμFA (≥ +0.10 change) had limited ΔFA: 3 with small decreases, 2 with no change, and 2 with small increases.
Discussion
This first study of acute stroke using b-tensors demonstrates increased µFA in lesion WM over all patients scanned ~1 day after stroke onset, in contrast to the reduced typical FA. The elevated µFA in acute ischemia contrasts with its reduction in other clinical brain disorders such as tumor4-6, multiple sclerosis9, and age-related white matter lesions7. Higher µFA in acute ischemic lesion WM may reflect an increase in water ‘trapped’ within the anisotropic axons, while increased isotropic diffusional variance (MKI) may reflect cytotoxic edema related axon membrane beading and constrictions15 and the creation of multiple compartments with distinct diffusion characteristics. Membrane constrictions may also play a role in µFA increase. However, simulation has predicted a µFA decrease with membrane beading16, not an increase as we observe experimentally (Figure 3C). As a limitation, the impact of diffusion time on the measurements of b-tensor encoding in cerebral ischemia remains to be explored.In conclusion, multidimensional b-tensor encoding is clinically feasible with a rapid acquisition protocol, and the new metrics it enables provide new insight into the microstructural changes after acute stroke.
Acknowledgements
Grant support was provided by the Heart and Stroke Foundation of Canada.References
[1] Lawrenz M, Finsterbusch J. Double wave vector diffusion-weighted imaging reveals microscopic diffusion anisotropy in the living human brain. Magnetic Resonance in Medicine 69, 1072 (2013).
[2] Lasic S, Szczepankiewicz F, Eriksson S, Nilsson M, Topgaard D. Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Frontiers in Physics 2, 11, 1 (2014).
[3] Westin CF, Knuttson H, Pasternak O, Szczepankiewicz F, Ozarslan E, van Westen D, Mattisson C, Bogren M, O’Donnell L, Kubicki M, Topgaard D, Nilsson M. Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage 135, 345 (2016).
[4] Szczepankiewicz F, Lasic S, van Westen D, Sundgren P, Englund E, Westin CF, Stahlberg F, Latt J, Topgaard D, Nilsson M. Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: applications in healthy volunteers and in brain tumors. Neuroimage 104, 241 (2015).
[5] Szczepankiewicz F, van Westen D, Englund E, Westin CF, Stahlberg F, Latt J, Sundgren P, Nilsson M. The link between diffusion MRI and tumor heterogeneity: mapping cell eccentricity and density by diffusional variance decomposition. Neuroimage 142, 522 (2016).
[6] Nilsson M, Szczepankiewicz F, Brabec J, Taylor M, Westin CF, Golby A, van Westen D, Sundgren P. Tensor-valued diffusion MRI in under 3 minutes: an initial survey of microscopic anisotropy and tissue heterogeneity in intracranial tumors. Magnetic Resonance in Medicine in press (2019).
[7] Lampinen B, Szczepankiewicz F, Noven M, van Westen D, Hansson O, Englund E, Martensson J, Westin CF, Nilsson M. Searching for the neurite density with diffusion MRI: challenges for biophysical modeling. Human Brain Mapping 40, 2529 (2019).
[8] Lampinen B, Zampeli A, Björkman-Burtscher I M, Szczepankiewicz F, Källén K, Compagno Strandberg M, Nilsson M. Tensor-valued diffusion MRI differentiates cortex and white matter in malformations of cortical development associated with epilepsy. Epilepsia 61, 1701 (2020).
[9] Andersen K W, Lasič S, Lundell H, Nilsson M, Topgaard D, Sellebjerg F, Szczepankiewicz F, Siebner H R, Blinkenberg M, Dyrby T B. Disentangling white-matter damage from physiological fiber orientation dispersion in multiple sclerosis. Brain Communications. (2020).
[10] Basser P J, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical journal 66, 259 (1994).
[11] Sjölund J, Szczepankiewicz F, Nilsson M, Topgaard D, Westin C F, Knutsson H. Constrained optimization of gradient waveforms for generalized diffusion encoding. Journal of Magnetic Resonance 261, 157 (2015).
[12] Szczepankiewicz F, Westin CF, Nilsson M. Maxwell-compensated design of asymmetric gradient waveforms for tensor-valued diffusion encoding. Magnetic Resonance in Medicine 82, 1424 (2019).
[13] Szczepankiewicz F, Sjölund J, Ståhlberg F, Lätt J, Nilsson M. Tensor-valued diffusion encoding for diffusional variance decomposition (DIVIDE): Technical feasibility in clinical MRI systems. PloS one 14, e0214238 (2019).
[14] Nilsson M, Szczepankiewicz F, Lampinen B, Ahlgren A, de Almeida Martins A, Lasic S, Westin CF, Topgaard D. An open-source framework for analysis of multidimensional diffusion MRI data implemented in MATLAB. Proc. Intl. Soc. Mag. Reson. Med. (26), Paris, France, 2018. http://archive.ismrm.org/2018/5355.html
[15] Baron C, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C. Reduction of diffusion-weighted imaging contrast of acute ischemic stroke at short diffusion times. Stroke 46, 2136 (2015).
[16] Skinner N, Kurpad S, Schmit B, Budde M. Detection of acute nervous system injury with advanced diffusion-weighted MRI: a simulation and sensitivity analysis. NMR in Biomedicine 28, 1489 (2015).
Figures