0292
Pre- and post-neonatal in vivo DTI on mice: Targeting brain microstructures at 15.2T1Department of Chemical and Biological Physics, Weizmann institute of Science, Rehovot, Israel
Synopsis
DTI is a well-established technique for mapping brain microstructure. Brain’s microstructural features derive from white matter and change over the course of maturation; hence methods that can acquire DTI in utero and immediately post-partum, are of interest. The present study explores the use of a customized 3D phase-encoded Spatiotemporal Encoding (SPEN) MRI approach that can be used to overcome the motional and susceptibility challenges arising in such instances, delivering quality DTI volumetric data at 15.2T. Maps of ADC, MDD and FA could thus be collected for mice fetal brains in utero, as well as within the first week post-partum.
Introduction
Brain DTI is widely used to examine brain development and maturation, and can shed insight on normal and pathological processes – particularly when combined with mice models [1, 2]. In vivo examinations of these processes in fetal and neonatal mouse brains could be particularly informative tools in research, yet these are challenged by susceptibility artifacts, and by the impossibility to rigidly fix the animal’s head and thereby prevent physiological motions. Adding to these challenges is the smallness of the targets, which demand achieving high spatial resolutions for achieving a meaningful structural analysis; this poses an additional penalty on the already sensitivity-taxed diffusion acquisitions.We have recently demonstrated that interleaved 2D SPatiotemporal ENcoding (SPEN) sequences at 7T and 3D SPEN sequences at 15.2T could deliver quality maps of fetal and neonatal apparent diffusion coefficients (ADCs), respectively [3, 4]. This study explores the feasibility of porting these investigations to 3D DTI maps at 15.2 T, of mice brain in utero as well as within the first week of life.
Methods
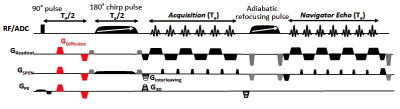
Animal handling: Pregnant and newborn C57BL/6 mice were scanned with protocols approved by a Weizmann Institute IACUC. During imaging, the newborn mice were induced by ~1-2.5% isoflurane and then kept under ~0.3-1% isoflurane –with the concentrations adjusted according to the mice’s age– mixed with 20/80 % O2/N2. For the pregnant mice, isofluorane anesthesia levels were kept at 1-2%. For post-partum animals respiration was monitored throughout via a pressure sensor and maintained at 35-60 breaths per minute; a customized bed for these newborn-sized mice was built to slightly restrain the bodies of these animals within the coil region. In all cases the body temperature was maintained using a water-based heating system.MRI scans and data analysis: 3D DTI SPEN sequences (Figure 1) were written and run within the Paravision 6 software of a 15.2 T Bruker scanner. Pregnant and newborn animals were studied using body-volume and surface cryocooled transceiver coils, respectively. The sequence included interleaving along the SPEN axis, phase-encoding along the 3rd dimension, and a final 2D echo-based reacquisition in the readout/SPEN domain for all interleaves and phase-encodes, to correct for phase distortions arising from minor motions [5]. Acquisitions were also implemented the spin-echo EPI with double-sampling DTI sequence provided by the scanner. Experiments were run with the following parameters: TR/TE=1000/30 ms; field of views ≈ 32x32x10 mm for the pregnant animals, 12x12x6 mm for the newborns; spatial resolutions ≈ 160µm isotropic for the pregnant animals, 70x70x100 µm for the newborns; 5 interleaved SPEN segments; 8-10 diffusion directions; 2 averages; a single b-value (≈800-950 mm/s²). Total acquisition times were always restrained to 2 hours. After denoising the images, the diffusion tensor was then computed on Matlab, and Fractional Anisotropy (FA), Apparent Diffusivity (ADC) and color-coded Main Diffusion Direction (MDD) maps highlighting the brain structures, were computed from the diffusivity tensor.
Results
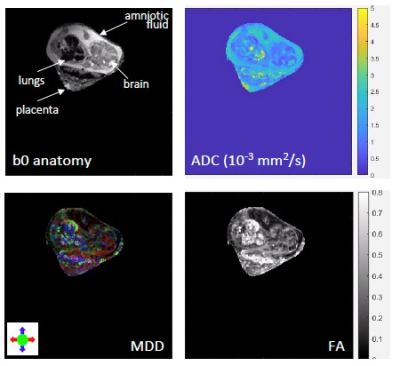
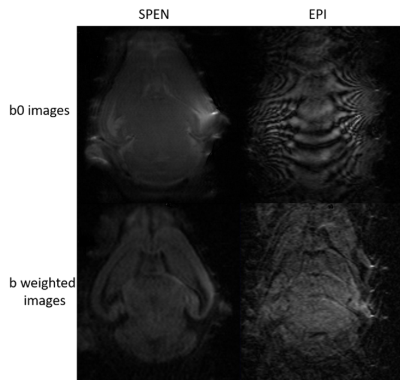
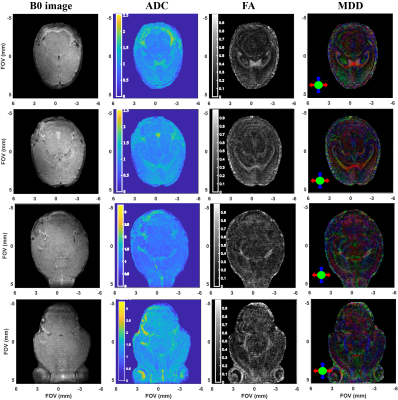
Figure 2 summarizes the kind of diffusion-based measurements that were obtained on scanning live fetuses in utero. Shown are b0 (SPEN) images of the dam’s abdomen focusing on a single fetoplacental unit 15 days into the pregnancy, together with the corresponding FA, ADC and MDD maps. These images show the targeted unit masked for further clarity. While these (and similar) data are still under analysis, it shows the lack of directionality in the faster-diffusing regions of the fetal brain, and the presence of well-specified directions in white matter regions. Figure 3 illustrates another aspect of this study, with b0 and b-weighted 3D SPEN and 3D spin-echo EPI slices extracted from 3D acquisition on 2-days-old pups, focusing on the animal brain. The differences in the susceptibility distortions introduced by the ear channels, the sinuses and the buccal cavity on the images delivered by SPEN and spin-echo EPI, are evident. Figure 4 illustrates a series of SPEN-derived multi-slice images and maps, measured on a 4-days-old mice. Although some minor artifacts are observable as stripes in these data, quality bo images and detailed ADC, FA and MDD data and make it possible to anatomically define different layers structures that match well with ex-vivo atlas information.Conclusions
This study presents initial high-resolution results of in utero and of neonatal brain diffusion tensor imaging investigations on mice, recorded at 15.2T. SPEN appears capable of overcoming susceptibility distortions and other non-idealities that tend to corrupt these data, leading to high-definition DTI insight about brain development in vivo. Ongoing research includes analyzing the age maturation evidenced by these data as reflected by changes in the ADC, MDD and FA parameters. Methodological efforts are also ongoing to speed up the acquisitions via compressed sensing methods thereby avoiding motional instabilities, and incorporate post-acquisition motion-correction algorithms.Acknowledgements
Support from the Minerva Foundation (Germany), the Israel Science Foundation, and the Clore and Kimmel Institutes for Magnetic Resonance (Weizmann Institute), are acknowledged.References
[1] Chahboune H, Ment LR, Stewart WB, et al. Neurodevelopment of C57B/L6 mouse brain assessed by in vivo diffusion tensor imaging. NMR Biomed 2007; 20: 375–382.
[2] Larvaron P, Boespflug-Tanguy O, Renou J-P, et al. In vivo analysis of the post-natal development of normal mouse brain by DTI. NMR Biomed 2007; 20: 413–421.
[3] Bao Q, Liberman G, Solomon E, et al. High-resolution diffusion MRI studies of development in pregnant mice visualized by novel spatiotemporal encoding schemes. NMR Biomed; 33. DOI: 10.1002/nbm.4208.
[4] Yon M, Bao Q, Chitrit OJ, et al. High-Resolution 3D in vivo Brain Diffusion Tensor Imaging at Ultrahigh Fields: Following Maturation on Juvenile and Adult Mice. Front Neurosci 2020; 14: 590900.
[5] Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 2009; 62: 468–475.
Figures