0281
xSPEN spectroscopy: a self-navigated fast chemical shift encoded echo planar imaging acquisition1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Departments of Nuclear Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Synopsis
xSPEN is a single-shot echo-planar imaging-based MRI approach with exceptional resilience to chemical shifts and field inhomogeneities. We introduce a time increasing (t1) evolution as chemical shift encoding to fast obtain multiple-voxel spectroscopy. The new method endows a 2D self-navigated motion correction and a unique J-decoupled spectrum by splitting the t1 evolution into τ-t1/2 and t1/2 on two sides of π pulse, which results a constant-τ J coupling evolution. We present in vitro results, demonstrating an alternative fast MRSI and increasing chemical shift separation and detection with the robustness to the in-plane motion and the unique J-decoupled spectrum capability.
Introduction
Echo Planar Spectroscopic Imaging (EPSI) significantly accelerates MRSI by simultaneous encoding of one spatial and the temporal dimension with echo-planar fast gradient oscillation during signal read-out [1]. However, only half spectral bandwidth is used in conventional EPSI, as the even and odd readout echoes are utilized separately during reconstruction. Limited spectral bandwidth is a problem particularly at higher magnetic field strengths (>3 T), as chemical field dispersion effects increase, leading to spectral aliasing [2]. Besides, the splittings because of J couplings result a broader peak along the chemical shift axis, which make coupled compounds such as GABA and glutamate harder to detect and quantify [3, 4]. Recently, cross-term spatiotemporal encoding (xSPEN) [5] is an introduced imaging approach delivering single-scan 2D images with exceptional resilience to chemical shifts and field inhomogeneities. The shot-to-shot distortion-free xSPEN images endow robustness to in-plane motion. Here, we introduced a time increasing (t1) evolution as chemical shift encoding to fast obtain multiple-voxel spectroscopy. This xSPEN spectroscopy has no limitation of spectral bandwidth while the echo-planar imaging acquisition grants a high sampling efficiency of MRSI. Furthermore, we demonstrate in vitro results with an increasing chemical shift separation and detection by taking advantage of the unique J-decoupled spectrum capability of this technique.Methods
The xSPEN spectroscopy sequence is shown in Fig. 1c by performing multiple scans with increasing time (t1) evolution while the echo-planar imaging-based acquisition of xSPEN grants a high sampling efficiency of MRSI. The chemical shift spectrum of each voxel is obtained by the Fourier transform along the time-increasing dimension. The resolution and bandwidth of the chemical shift spectrum will be determined by the usual Nyquist criteria of the maximum t1 and the t1 step, which can be flexibly adapted. By splitting the t1 evolution into τ-t1/2 and t1/2 on two sides of π pulse, it results a constant-τ J coupling evolution bringing a J-decoupled xSPEN spectroscopy sequence as shown in Fig.1d. This unique J-decoupled xSPEN spectroscopy increases the chemical shift separation and detection of coupled compounds. Besides, the single-shot distortion-free xSPEN images allow a self-navigated in-plane motion correction. Studies were conducted with a 3.0T United Imaging uMR790 system (Shanghai, China). To demonstrate the concept, a phantom of ~200 mM GABA aqueous solution with three different multiplets correspond to the three methylene (CH2) groups in the GABA molecule. Parameters for the EPSI include TR =2 s, 200×200 mm2 FOV, 10 mm slice thickness, 80×80 image matrix size was cropped into 32×32 for the comparison, the echo spacing is 0.53 ms. Parameters for the xSPEN spectroscopy include TR =2 s, 200×200 mm2 FOV, 10 mm slice thickness, 32×32 image matrix size, a total of 160 t1 encodings with two bandwidths of 1000 Hz and 2000 Hz. The constant J coupling evolution τ=120 ms is used for J-decoupled xSPEN spectroscopy. For the robustness to motion study, factitious phantom motions were introduced during the xSPEN spectroscopy scanning with/without water suppression.Results and Discussion
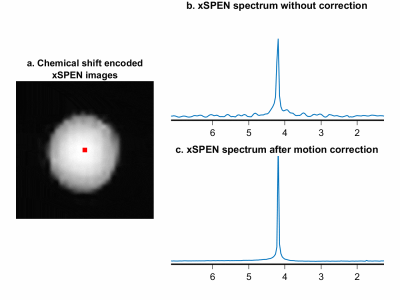
Figure 2 shows the comparison among EPSI, xSPEN spectroscopy, and J-decoupled xSPEN spectroscopy. The splittings because of J couplings in the EPSI and xSPEN spectroscopy result broader peaks along the chemical shift axis, while high-resolution singlets of J-decoupled xSPEN spectroscopy enhance the chemical shift separation and detection. A larger spectral bandwidth of 2000 Hz can be easily achieved by xSPEN spectroscopy sequences as shown in Fig.2b. Both the field inhomogeneities and J couplings can result spectral broadening, which may lead to inaccurate chemical shift maps as shown in Fig. 3 while the J-decoupled xSPEN spectroscopy can benefit the quantification by simplifying the coupled compounds. Figure 4 demonstrates the robustness of xSPEN spectroscopy to the in-plane motion, shot-to-shot chemical shift encoded xSPEN images allow a self-navigated motion tracking and correction to greatly reduce the spectral distributions.Conclusions
We propose an alternative fast magnetic spectroscopy imaging termed as xSPEN spectroscopy endowed with flexible spectral bandwidths, a unique J-decoupled spectrum capability and the robustness to in-plane motion.Acknowledgements
This work is supported by National Science Foundation of China (No. 62001290) and sponsored by Shanghai Sailing Program (20YF1420900).References
[1] Posse S, Otazo R, Dager SR, Alger J. MR spectroscopic imaging: principles and recent advances. J Magn Reson Imaging 2013;37(6):1301-1325.
[2] Al-Iedani O, Lechner-Scott J, Ribbons K, Ramadan S. Fast magnetic resonance spectroscopic imaging techniques in human brain- applications in multiple sclerosis. J Biomed Sci 2017;24(1):17.
[3] Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc 2012;60:29-41.
[4] Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 2013;26(12):1630-1646.
[5] Zhang Z, Seginer A, Frydman L. Single-scan MRI with exceptional resilience to field heterogeneities. Magn Reson Med 2017;77(2):623-634.
Figures