0269
Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is associated with lower R2 relaxation rate1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is now recognized as a common age-related neuropathology that has been linked to cognitive decline and dementia. In this work, the spatial pattern of R2 alterations associated with LATE-NC was investigated in a large (N=797) community cohort of older adults. Voxel-wise analysis revealed a pattern of lower R2 for greater LATE-NC burden, controlling for all other neuropathologies and demographics. This pattern involved mainly the temporal, frontal, occipital lobes and basal ganglia. To our knowledge this is the first R2 investigation in LATE-NC.
Introduction
Limbic predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is a recently recognized disease entity referring to the accumulation of transactive response DNA binding protein of 43kDa (TDP-43) proteinopathy in older adults. LATE-NC is detected at autopsy in 20-50% of individuals older than 80 years of age. LATE-NC is associated with lower memory, cognitive decline and increased likelihood of dementia above and beyond the contributions of other age-related neuropathologies1–5. Moreover, LATE-NC has been shown to account for nearly as much of the variance in late-life cognitive decline as neurofibrillary tangles (hallmark pathology of Alzheimer’s)6. Thus, LATE-NC is now recognized as a common and deleterious neuropathology of the aging brain. In this work, we hypothesized that neuronal changes caused by LATE-NC may be detected through alterations in R2 relaxation rate, similar to the effects of other neurodegenerative pathologies such as Alzheimer’s7,8. Therefore, the goal of this study was to extract the spatial pattern of R2 alterations associated with LATE-NC in a large community cohort of older adults.Methods
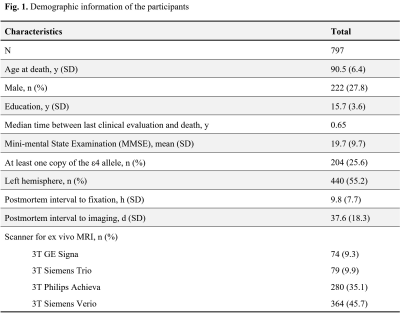
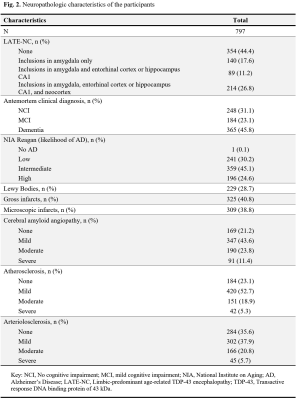
Cerebral hemispheres were obtained from 797 deceased older adults participating in three longitudinal, clinical-pathologic cohort studies of aging9,10: the Rush Memory and Aging Project (MAP), the Religious Orders Study (ROS), and the Minority Aging Research Study (MARS) (Fig. 1). Hemispheres were imaged ex-vivo on 3T clinical MRI scanners, while immersed in 4% formaldehyde solution, using a 2D spin-echo sequence with multiple echo times ranging from 11-83 ms and 0.6×0.6×1.5 mm3 voxel size. The R2 relaxation rate was quantified voxel-wise by fitting a monoexponential decay function S = S0∙exp(-R2∙t) to the multi-echo spin-echo data. The images from one of the echoes were non-linearly registered to an ex-vivo MRI brain template using ANTS11 and the transformations were applied to the R2 maps to enable voxel-wise analysis7,12. Following ex-vivo MRI, hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to all clinical and imaging findings (Fig. 2).Voxel-wise linear regression was used to investigate the association of R2 relaxation rate with LATE-NC controlling for other neuropathologies, including Alzheimer’s pathology, Lewy bodies, gross and microscopic infarcts, atherosclerosis, cerebral amyloid angiopathy, arteriolosclerosis, as well as age at death, sex, years of education, postmortem interval to fixation and postmortem interval to imaging, and scanner. The analysis was performed using the PALM tool (FMRIB, Oxford, UK)13 with no acceleration, 10,000 permutations, threshold-free cluster enhancement, and family-wise error correction for multiple comparisons. Statistical significance was set at p<0.05.
Results and Discussion
Voxel-wise analysis demonstrated lower R2 for greater LATE-NC burden in a number of brain regions in the temporal, frontal, occipital lobes and basal ganglia (Fig. 3). The strongest effects were observed in the amygdala, hippocampus, and neighboring temporal lobe regions. These findings are in agreement with previous work showing that LATE-NC typically begins and is most commonly seen in the amygdala14,15. In later stages, LATE-NC is deposited in the hippocampus and entorhinal cortex, and then the neocortex and other regions, especially in the frontal lobe4 where we also saw R2 shortening with LATE-NC. In addition, lower R2 with greater LATE-NC burden was demonstrated in the caudate, insular and occipital white matter regions which have been studied less in terms of LATE-NC pathology14,16,17. No brain regions showed positive associations of R2 with LATE-NC.To the best of our knowledge, this study is the first to reveal the spatial pattern of R2 alterations linked to LATE-NC. The findings of this work suggest that LATE-NC has an independent contribution to R2 shortening above and beyond the contributions of other neuropathologies and demographics. The extracted pattern of R2 shortening is rather consistent with the known distribution of LATE-NC in the brain of older adults. This pattern may potentially aid in in-vivo prediction of this devastating neuropathology which, currently, can only be diagnosed at autopsy.
The present study combined MRI and pathology data from a particularly large number of community-based older adults which enhances the robustness and generalization of the findings. Although MRI was conducted ex-vivo, we expect the results to hold in-vivo since we have previously demonstrated that ex-vivo R2 values are linearly linked to in-vivo R2 values for the tissue preparation and imaging protocol used here7.
Conclusion
The present study demonstrated a spatial pattern of lower R2 relaxation rate for greater LATE-NC burden in a large community cohort of older adults. This pattern involved mainly the temporal, frontal, occipital lobes and basal ganglia, and the strongest effects were observed in the amygdala, hippocampus, and neighboring temporal lobe regions. The link between R2 and LATE-NC presented in this work may be exploited towards the development of algorithms for the prediction of this devastating, recently recognized disease entity.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2-UH3NS100599
National Institute on Aging (NIA) R01AG064233
National Institute on Aging (NIA) R01AG067482
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) R01AG015819
National Institute on Aging (NIA) RF1AG022018
National Institute on Aging (NIA) R01AG056405
National Institute on Aging (NIA) P30AG010161
References
- Kawas C, Corrada M. Alzheimers and Dementia in the Oldest-Old: A Century of Challenges. Curr Alzheimer Res 2006;3(5):411–419.
- Tremblay C, St-Amour I, Schneider J, et al. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 2011;70(9):788–798.
- Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 2014;127(6):811–824.
- Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019;142(6):1503–1527.
- Robinson JL, Porta S, Garrett FG, et al. Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 2020;143(9):2844–2857.
- Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013;70(11):1418–1424.
- Dawe RJ, Bennett DA, Schneider JA, et al. Ex vivo T2 relaxation: Associations with age-related neuropathology and cognition. Neurobiol Aging 2014;35(7):1549–1561.
- Yu L, Dawe RJ, Buchman AS, et al. Ex vivo MRI transverse relaxation in community based older persons with and without Alzheimer’s dementia. Behav Brain Res 2017;322:233–240.
- L. Barnes L, C. Shah R, T. Aggarwal N, et al. The Minority Aging Research Study: Ongoing Efforts to Obtain Brain Donation in African Americans without Dementia. Curr Alzheimer Res 2013;9(6):734–745.
- Bennett DA, Buchman AS, Boyle PA, et al. Religious Orders Study and Rush Memory and Aging Project. J Alzheimer’s Dis 2018;64(s1):S161–S189.
- Avants B, Tustison N, Song G. Advanced Normalization Tools (ANTS). Insight J 2009;1–35.
- Dawe RJ, Yu L, Leurgans SE, et al. Postmortem MRI: a novel window into the neurobiology of late life cognitive decline. Neurobiol Aging 2016;45:169–177.
- Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage 2014;92:381–397.
- Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 2016;131(4):571–585.
- Nag S, Yu L, Wilson RS, et al. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 2017;88(7):653–660.
- Josephs KA, Murray ME, Tosakulwong N, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol 2019;137(2):227–238.
- Bejanin A, Murray ME, Martin P, et al. Antemortem volume loss mirrors TDP-43 staging in older adults with non-frontotemporal lobar degeneration. Brain 2019;142(11):3621–3635.
Figures