0266
Association of age-related neuropathologies with shape of subcortical structures in a large community cohort of older adults1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
Age-related neuropathologies have devastating effects on subcortical brain structures. MRI can be used to explore patterns of atrophy associated with various diseases, however, definitive diagnosis of age-related neuropathologies is only possible at autopsy. Therefore, this work combined ex-vivo MRI and detailed pathologic assessment (gold standard) in a large (N=814) community cohort of older adults to investigate the link between age-related neuropathologies and the shape of different subcortical brain structures. The resulting patterns of deformation may contribute towards development or enhancement of in-vivo classifiers of these neuropathologies.
Introduction
Age-related neuropathologies have devastating effects on subcortical brain structures. The various neurodegenerative and vascular pathologies have different patterns of accumulation in the brain, and are expected to lead to different patterns of atrophy1. Although numerous studies have used MRI to map the effects of Alzheimer’s on the shape of subcortical brain structures, Alzheimer’s is most often mixed with other, less studied but also detrimental neuropathologies that can only be diagnosed at autopsy. Therefore, the purpose of this work was to combine ex-vivo MRI and detailed neuropathology in a large community cohort of older adults to conduct the most comprehensive investigation to-date on the association of age-related neuropathologies with the shape of subcortical brain structures.Methods
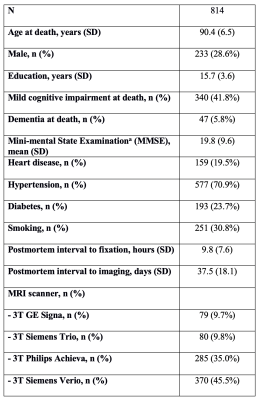
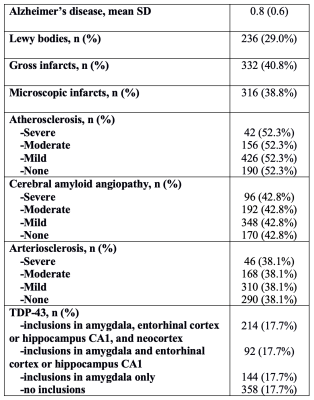
Participants and DataCerebral hemispheres were obtained from 814 participants of the Rush Memory and Aging Project2 (MAP) and Religious Orders Study3 (ROS), two longitudinal cohort studies of aging (Fig.1). A single brain hemisphere from each participant was imaged ex-vivo on a clinical 3T MRI scanner, while immersed in 4% formaldehyde solution. Following ex-vivo MRI, all hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to clinical and imaging findings (Fig.2).
Ex-vivo MR image processing
T2-weighted images from all hemispheres were used to segment the following subcortical brain regions: nucleus accumbens, amygdala, caudate, hippocampus, putamen, and thalamus. The quality of all segmentations was assessed by visual inspection following standardized protocols4. Since one hemisphere was imaged for each participant, right hemisphere segmentations were mirrored to appear like left hemisphere segmentations and combined with the rest of the left hemisphere segmentations for the subsequent shape and statistical analysis.
Shape estimation
Subcortical shape measures were computed using the ENIGMA-Shape protocol5. The Jacobian determinant derived from tensor-based morphometry6 (TBM) was calculated vertex-wise for all structures. The logarithm of the Jacobian determinant was used as a measure of shape morphometry indicating atrophy or expansion of a structure relative to the corresponding template.
Statistical analysis
Vertex-wise linear regression was performed for each subcortical structure, modeling the logarithm of the Jacobian determinant at each vertex (outcome variable) as a function of a comprehensive list of age-related neuropathologies, including Alzheimer’s pathology, transactive response DNA-binding protein 43 (TDP-43) pathology, Lewy bodies, arteriolosclerosis, atherosclerosis, gross and microscopic infarcts, and cerebral amyloid angiopathy, and controlling for age at death, sex, years of education, height (as a surrogate for intracranial volume), scanner, postmortem interval to fixation (PMI) and postmortem interval to imaging (PMII). P-value maps were corrected for multiple comparisons using a modified searchlight false discovery rate (FDR) procedure. Significance was set at p<0.05. Statistical analysis was performed using scripts from the ENIGMA-Git page7.
Results and Discussion
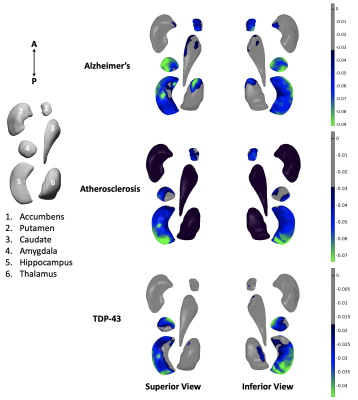
Results of shape analysis are shown in Figure 3. Alzheimer’s pathology, atherosclerosis, and TDP-43 pathology were all associated with unique patterns of inward deformation of the hippocampus and amygdala. More specifically, Alzheimer’s pathology was linked most strongly with atrophy in the anterior lateral of the hippocampus, and lateral and medial amygdala, atherosclerosis was linked more significantly to atrophy in the posterior one third of the hippocampus and lateral portions of the amygdala, and TDP-43 was linked to atrophy mainly in the posterior three quarters of the hippocampus, and mainly anterior medial portions of the amygdala. These findings for Alzheimer’s and TDP-43 pathology were in good agreement with our previous work8,9. The atherosclerosis patterns represent novel findings. Inward deformation of the nucleus accumbens was associated with Alzheimer’s pathology and atherosclerosis. Atrophy of the anterior and inferior lateral thalamus was mainly linked to Alzheimer’s pathology and TDP-43, respectively. Inward deformations of the medial aspect of the putamen and anterior caudate were associated with Alzheimer’s pathology.Lewy bodies, infarcts, arteriolosclerosis, and cerebral amyloid angiopathy did not show any links to the shape of subcortical brain structures. Also, no pathology was associated with expansion of any of the structures under study. Finally, although MRI was conducted ex-vivo, we expect the results to translate well to the in-vivo case, since we have previously demonstrated that, for the tissue preparation and imaging protocol used here, a linear relationship exists between structural brain information collected ex vivo and in vivo on the same individuals10.
Conclusion
The present work in a large community cohort of older adults represents the most comprehensive investigation to-date on the association of age-related neuropathologies with the shape of subcortical brain structures. Among the different age-related neuropathologies, Alzheimer’s pathology, TDP-43 pathology, and atherosclerosis are the ones that have independent contributions on shape abnormalities of subcortical brain structures. Although there were some similarities in the signatures of different neuropathologies on the shape of subcortical brain structures, there were also many differences that could potentially contribute towards in-vivo prediction of these neuropathologies.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2-UH3NS100599
National Institute on Aging (NIA) R01AG064233
National Institute on Aging (NIA) R01AG067482
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) R01AG015819
National Institute on Aging (NIA) P30AG010161
References
1. Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. Published online January 12, 2017:aww341. doi:10.1093/brain/aww341
2. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663. doi:10.1523/JNEUROSCI.3593-07.2007.Omega-3
3. Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2013;33 Suppl 1:S397-403. doi:10.3233/JAD-2012-129007
4. http://enigma.ini.usc.edu/protocols/imaging-protocols/.
5. http://enigma.usc.edu/ongoing/enigma-shape-analysis/.
6. Gutman BA, Jahanshad N, Ching CRK, et al. Medial demons registration localizes the degree of genetic influence over subcortical shape variability: An N&#x003D; 1480 meta-analysis. In: 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI). IEEE; 2015:1402-1406. doi:10.1109/ISBI.2015.7164138
7. https://github.com/ENIGMA-git/ENIGMA/tree/master/WorkingGroups.
8. Hanko V, Apple AC, Alpert KI, et al. In vivo hippocampal subfield shape related to TDP-43, amyloid beta, and tau pathologies. Neurobiol Aging. 2019;74:171-181. doi:10.1016/j.neurobiolaging.2018.10.013
9. Makkinejad N, Schneider JA, Yu J, et al. Associations of amygdala volume and shape with transactive response DNA-binding protein 43 (TDP-43) pathology in a community cohort of older adults. Neurobiol Aging. 2019;77:104-111. doi:10.1016/j.neurobiolaging.2019.01.022
10. Kotrotsou A, Bennett DA, Schneider JA, et al. Ex vivo MR volumetry of human brain hemispheres. Magn Reson Med. 2014;71(1):364-374. doi:10.1002/mrm.24661
Figures