0262
The aging quantitative brain: a multiparametric qMRI study1INM-4, Research Centre Juelich, Juelich, Germany, 2Faculty of Medicine, JARA, RWTH Aachen University, Aachen, Germany, 3INM-11, JARA, Research Centre Juelich, Juelich, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
Quantitative MRI parameters are determined by the properties of tissue on a microscopic scale and can be expected to reflect microstructural changes created by aging. Here, we investigate a multiparametric qMRI signature of healthy ageing on 26 healthy volunteers, characterised in a high-dimensional parametric space (water content, relaxometry, qMT). Changes with age in the mean values and correlations between parameters are observed and interpreted.
Introduction
The proportion of the world’s population aged 60 or more is predicted to double by 2055 compared to the year 2000 [1]. This change in demographics naturally generates huge interest in understanding healthy and pathological brain aging. MRI plays a major role in characterizing these changes in vivo. In addition to providing measures of macroscopic morphological changes, MRI delivers quantitative parameters which are determined by the properties of tissue on a microscopic (~10um) scale [2]. Microstructural signatures of changes in aging brain tissue can thus be provided in vivo by quantitative MRI [3,4,5]. Here, we investigate a multiparametric qMRI signature of healthy ageing on 26 healthy volunteers, characterised in a high-dimensional parametric space: water content (H2O); R1 and R2* relaxometry; qMT parameters fbound, kex and MTR; QSM; cortical thickness and local gyrification index, as well as coefficients characterizing linear dependencies between pairs of parameters.Materials and methods
A quantitative MRI protocol was measured from a subset of ~150 volunteers among those included in the population-based cohort study 1000BRAINS [6], conducted on a 3T Tim-Trio scanner. Data on twenty-six volunteers covering 7 decades of age as uniformly as possible (between 27 and 80 yo, mean age 53±16 years, male/female 19/7) and believed to represent healthy aging were selected for analysis. Exclusion criteria were pathological structure changes visible on MPRAGE or FLAIR (except age-related changes), known medication intake, a reported history of diseases affecting the central nervous system, hypertension and diabetes. Details of the acquisition have been reported in [7,8] on different sub-collectives selected from the 1000BRAINS cohort. Briefly, quantitative mapping is based on four 3D multiple-echo (18 echoes) gradient echo scans, TR=50ms, flip angles of 7deg and 40deg, each with and without MT preparation, complemented by flip angle mapping using AFI [9]. Whole-brain coverage with 1x1x2mm3 resolution is achieved in less than 20 minutes. Each pair of scans with 7 and 40deg flip angle (one with, one without MT) provides input for M0, R1 and R2* mapping (with and w/o MT) as described in [10]. Under the assumption of complete saturation of the bound proton pool and negligible direct saturation to the mobile water pool, quantitative MT parameters can be derived [2]: bound proton fraction (fbound), magnetization exchange rate (kex), and M0 reduction caused by the MT pulse (MTR). Phase information from each 3D multi-echo scan provides input for quantitative susceptibility mapping (QSM). The MPRAGE anatomical scan included in the 1000Brains imaging protocol [4] was used for brain parcellation according to the Destrieux atlas using Freesurfer [11]. The information was transferred to the space of the quantitative maps using ANTs registration. Mean values of the quantitative parameters were calculated for each ROI. Correlations between pairs of parameters were described within each ROI by a linear model ([P1] = beta0(P1) + beta1(P1,P2)[P2]) and the Pearson correlation coefficient. For the combination of parameters QSM, R1 and R2*, an extended linear model was tested [QSM] = beta0 + beta1[R1] + beta2[R2*], reflecting the interplay of myelin and iron in determining QSM and R2* [12], while myelin is the main determinant of R1 [Koenig]. The alteration of each calculated parameter with age was assessed using a similar linear regression approach and partial Pearson correlation including the subjects’ sex as covariate: [qMRI] = beta0 + beta1[age] + beta2[sex]. Freesurfer-calculated cortical thickness (CT) and ROI volumes normalized by the total intracranial volume (TIV) were included in the analysis. The statistical analysis was done in Python (statsmodels and Pingouin packages). Outlier exclusion was performed using a density-based clustering approach (DBSCAN) as implemented in scikit-learn. In order to investigate WM-related changes in more detail, the quantitative maps from all subjects were nonlinearly registered to the MNI template.Results
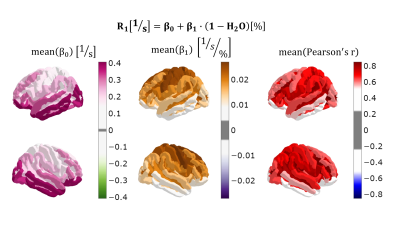
The results are summarized in Figs. 1-3, describing correlations between quantitative parameters (R1 and (1-H2O) are chosen as example), as well as the effect of aging on the mean values and correlations between parameters (exemplary results chosen).Discussion and Conclusions
Given the complexity of the information, we can address here only a few parameters and ROIs. Between pairs of parameters, R1 and fbound showed the highest correlations. Indeed, the bound protons which exchange magnetization with mobile water (fbound) are also those influencing the observed relaxation rate. 1-H2O and fbound and 1-H2O and R1 were also well correlated, showing that a significant fraction of macromolecules contribute to MT and relaxation effects. We discuss in more detail the changes in the inferior parietal lobule (arrows in Fig4). Decreasing d(1-H2O)/d(fbound) means that less macromolecules contribute to MT. These are most probably intracellular macromolecules, which contribute more to MT. Consequently, R1 decreases, as observed, because its sources of relaxation disappear. QSM decreases, which could be because iron (also previously intracellular) is transported out of this region. Myelin transport out of the region, as well as myelin fragmentation are expected to _increase_ susceptibility (the latter found e.g. in MS lesions, [16]). The source of the qMRI changes detected in the inferior parietal lobule appears to be neuronal loss and transport of iron and macromolecules from the region. In conclusion, quantitative parameters appear to reflect several aspects of tissue microscopy and changes with age, indicating high potential for insight into aging mechanisms.Acknowledgements
AMO-P and NJS acknowledge support by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 764513. Support from Mr. Ricardo Loucao, Dr. Elene Iordanishvili and Dr Melissa Schall in the early stages of this work is gratefully acknowledged.References
[1] World Health Organization. Multisectoral action for a life course approach to healthy ageing: draft global strategy and plan of action on ageing and health. 69th World Health Assembly, Geneva, 2016 April 22 (A69/17). Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_17-en.pdf.
[2] P. Tofts, Ed., Quantitative MRI of the brain: measuring changes caused by disease. Chichester, West Sussex ; Hoboken, NJ: Wiley, 2003
[3] Callaghan, M. F. et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol. Aging 35, 1862–1872 (2014).
[4] Seiler, Alexander et al. “Cortical aging - new insights with multiparametric quantitative MRI.” Aging vol. 12,16 (2020): 16195-16210. doi:10.18632/aging.103629
[5] Filo, S., Shtangel, O., Salamon, N. et al. Disentangling molecular alterations from water-content changes in the aging human brain using quantitative MRI. Nat Commun 10, 3403 (2019). https://doi.org/10.1038/s41467-019-11319-1
[6] Caspers S, Moebus S, Lux S, Pundt N, Schutz H, Muhleisen TW, Amunts K (2014) Studying variability in human brain aging in a population-based German cohort-rationale and design of 1000BRAINS. Front Aging Neurosci 6:149
[7] Schall, Melissa et al. 2020 Increasing body mass index in an elderly cohort: Effects on the quantitative MR parameters of the brain. J. Magn. Reson. Imaging 51:514–523.
[8] Iordanishvili E, Schall M, Loução R, Zimmermann M, Kotetishvili K, Shah NJ, Oros-Peusquens AM. Quantitative MRI of cerebral white matter hyperintensities: A new approach towards understanding the underlying pathology. Neuroimage. 2019 Nov 15;202:116077.
[9] V. Yarnykh Magn Reson Med. 2007 Jan;57(1):192-200.
[10] Schall, Melissa et al. 2018 “A 3D two-point method for whole-brain water content and relaxation time mapping: Comparison with gold standard methods.” PloS one vol. 13,8 e0201013.
[11] http://surfer.nmr.mgh.harvard.edu/, Freesurfer version 6.0.0
[12] Hametner et al 2018. Neuroimage. 2018;179:117-133.
[13] S. Koenig, MagnResonMed 1990, https://doi.org/10.1002/mrm.1910200210
[14] Fatouros et al 1991, Magn ResonMed 1991, Feb;17(2):402-13. doi: 10.1002/mrm.1910170212.
[15] H. Neeb, K. Zilles, N.J. Shah 2006. NeuroImage 29(3):910-22. doi:10.1016/j.neuroimage.2005.08.062
[16] K. Deh et al. J Magn Reson Imaging. 2018;48(5):1281-1287. doi:10.1002/jmri.25997
Figures