0261
Cumulative effects of a statin cocktail on cerebral blood flow and cognitive function in patients with Alzheimer’s Disease1Department of Radiology, University of Massachusetts Medical School, Worcester, MA, United States, 2Department of Neurology, University of Massachusetts Medical School, Worcester, MA, United States, 3Department of Psychiatry, University of Massachusetts Medical School, Worcester, MA, United States
Synopsis
Decreased cerebral metabolism has been implicated in pathogenesis of Alzehimer’s disease. The endothelial nitric oxide synthase (eNOS) pathway plays a major role in cerebral blood flow regulation. This study used DSC-MRI in 10 patients treated with a drug regimen for supporting the eNOS pathway to investigate how perfusion patterns were associated with treatment response based on clinical psychometrics. Regional analysis on rCBF maps showed significant signal changes in the cognitively improved cohort based on ADAS-cog scores while patients that showed cognitive deterioration or no change based on ADAS-cog scores did not show significant rCBF changes.
Introduction
Alzheimer’s disease (AD) affects the cognitive function of elderly people. The precise etiology of this degenerative disorder remains unknown and can be characterized by progressive dementia. Despite extensive research, no disease modifying treatment is available to treat AD. Vascular disease is a known risk factor for AD, and AD vessels are often found to be atrophic at autopsy. Studies have shown that a decrease in cerebral metabolism and blood flow are typical characteristics in AD patients.1,2 Thus, therapies aimed at supporting the structural and functional integrity of the brain’s microvascular endothelium could be a promising target to improve cerebral perfusion and cognition in AD.3 The endothelial nitric oxide synthase (eNOS) pathway upregulation in particular is known to support microvascular endothelial health.4-6 Whether upregulating the e-NOS pathway may impact AD outcome is uncertain. In this prospective, open-label, single group assignment, single center pilot study, we used perfusion MRI in patients with mild AD (Mini Mental State Examination [MMSE] score of 15-267) to explore whether the combined treatment with drugs known to augment the eNOS pathway increased cerebral blood flow in the brain.Methods
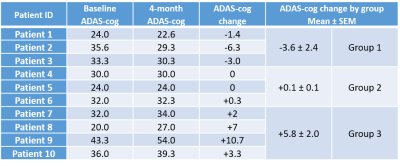
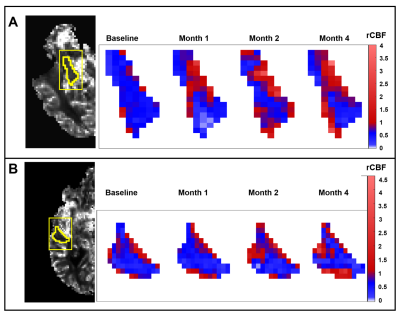
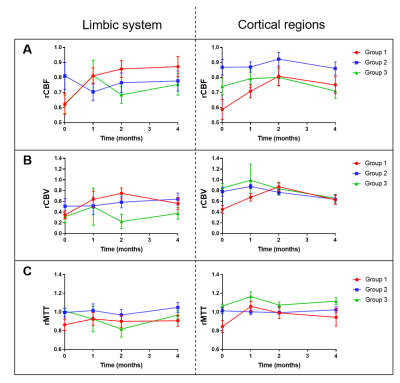
Eleven subjects with mild AD were from the dementia clinic at the University of Massachusetts Memorial Medical Center and monitored over a 4-month period. Patients were sequentially treated with the HMG-CoA reductase synthesis inhibitor simvastatin (which increases eNOS activity, months 0-4), L-Arginine (a substrate for nitric oxide in the eNOS pathway, months 1-4); and tetrahydrobiopterin (critical cofactor in the eNOS pathway, months 2-4). For each patient, cognitive function was evaluated using the Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-cog)8 to assess severity of cognitive impairment in multiple domains with higher scores showing more impairment. The MMSE was used to assess cognitive function where higher scores indicate better cognition. Dynamic susceptibility contrast (DSC-) MRI was performed with gadolinium to assess brain perfusion at 4 timepoints after initiation of the treatment regimen: baseline, 1-month, 2-months, and 4-months. Imaging protocol included DSC-MRI (TR/TE=1700/40ms, FA=75o, 100 dynamics, matrix=128×128) and T1-MPRAGE (TR/TE=7/3ms, FA=8o, matrix=256×256). MMSE scores were collected at each time-point prior to imaging sessions and ADAS-cog scores were collected at baseline and 4-month time-point. The ADAS-cog scores can be clinically relevant when they change by a magnitude of three points.11 Based on this criteria, the patient groups in our study were assigned (Fig. 1) as showing improvement (decline in ADAS-cog: Group 1), showing no change (no change in ADAS-cog: Group 2), and showing deterioration (increase in ADAS-cog: Group 3). The MRI perfusion parameters were grouped according to the three patient groups and subsequently analyzed to determine if there were any effects due to the drug regimen over the 4-month period.Image analysis was performed using ImageJ and DSC-MRI toolbox in MATLAB with semi-automated arterial input function selection and deconvolution algorithms.9,10 Cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) maps were generated for each patient at each time-point. To ensure objective comparison of CBF maps between time-points and across all patients, relative CBF (rCBF) maps were calculated by normalizing the CBF maps relative to the whole brain CBF. CBV and MTT maps were also normalized to generate relative CBV (rCBV) and relative MTT (rMTT) maps. Additionally, we compared rCBF differences between the groups by performing two-sample t-tests using the SPM12 software (Statistical Parametric Mapping). Then, regional analysis was performed by selecting regions of interest (ROI) in the limbic system (amygdala and hippocampus) and the cortical area (inferior parietal, middle frontal, and middle temporal lobes) and comparing the rCBF, rCBV, and rMTT values across the 4 time-points for the 3 patient groups. Analysis of variance (ANOVA) for mixed models was used to determine if there were significant changes in the perfusion parameters across time and between groups for the limbic and cortical regions.
Results and Discussion
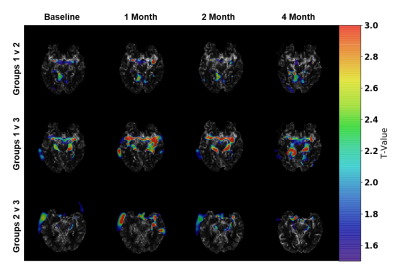
One patient was excluded from the study due to protocol violation. Included patients (67.1 ± 6.8 years) consisted of six females and four males. SPM analysis comparing the cognitively improved cohort (Group 1) to the cognitively declined cohort (Group 3) indicated greater blood flow in Group 1 as compared to Group 3 in the middle cerebral artery (MCA) bilaterally (higher T-values) with the progression of treatment (Fig. 2). By the 4th month, differences were attenuated when compared to previous time-points. The other group-wise comparisons showed no minimal differences. ROI analysis on the rCBF maps showed a significant signal increase in the limbic system and examined cortical regions in Group 1 patients relative to baseline indicating overall increased blood flow (Figs. 3 and 4). Groups 2 and 3 showed no significant changes in rCBF relative to baseline. rCBV significantly increased relative to baseline for Group 1 only, without significant change over time in Groups 2 and 3. rMTT showed no significant difference for all groups.Conclusion
Among mild AD patients treated with the statin cocktail, cognitive improvement over the 4-month study period was associated with an increase in the rCBF whereas no significant change was observed in subjects with unchanged or worsened cognition. Pending confirmation in a larger controlled trial, our observations suggest that rCBF-augmentation with medications known to act on the eNOS pathway may improve cognitive function in AD patients.Acknowledgements
Dr. Henninger is supported by K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. All other authors report no disclosures.References
1. Korte N, Nortley R, Atwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer's disease. Acta Neuropathol. 2020;140:793-810.
2. Postiglione A, Lassen NA, Holman BL. Cerebral blood flow in patients with dementia of Alzheimer's type. Aging (Milano). 1993;5(1):19-26.
3. Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of alzheimer's disease. Alzheimers Dement. 2014;10:372-380.
4. Fujii T, Onimaru M, Yonemitsu Y et al. Statins restore ischemic limb blood flow in diabetic microangiopathy via enos/no upregulation but not via pdgf-bb expression. Am J Physiol Heart Circ Physiol. 2008;294:H2785-2791.
5. Enomoto S, Sata M, Fukuda D, et al. Rosuvastatin prevents endothelial cell death and reduces atherosclerotic lesion formation in apoe-deficient mice. Biomed Pharmacother. 2009;63:19-26.
6. Strey CH, Young JM, Lainchbury JH, et al. Short-term statin treatment improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non-ischaemic heart failure. Heart. 2006;92:1603-1609.
7. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
8. Rosen WG, Mohs RC, Davis KL. A new rating scale for alzheimer's disease. Am J Psychiatry. 1984;141:1356-1364.
9. Francesca Z, Alessandra B, Gianluigi P, et al. Nonlinear stochastic regularization to characterize tissue residue function in bolus-tracking MRI: assessment and comparison with SVD, block-circulant SVD, and Tikhonov. IEEE Trans Biomed Eng. 2009;56(5):1287-1297.
10. Denis P, Alessandra B, Francesca Z, et al. Automatic selection of arterial input function on dynamic contrast-enhanced MR images. Comput Meth Prog Bio. 2011;104:e148-e157.
11. Schrag A, Schott JM. What is the clinically relevant change on te ADAS-Cog? J Neurol Neurosurg Psychiatry. 2012;83:171-173.
Figures