0249
Motion-robust, multi-slice, real-time MR PRFS Thermometry for MR-guided ultrasound thermal therapy in abdominal organs1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Radiation Oncology, University of California, San Francisco, San Francisco, CA, United States
Synopsis
MR temperature monitoring during ultrasound thermal therapy of abdominal organs is challenging due to respiratory motion. It is especially important for hyperthermia therapy, which requires accurate temperature measurements to ensure therapeutic heating within a narrow temperature window (37-45 °C). In this study we developed a motion-robust, multi-slice, real-time MR thermometry sequence and reconstruction pipeline for the monitoring of temperature in hyperthermia or thermal ablation therapy in abdominal organs. This technique was implemented in RTHawk and evaluated in simulated acquisitions, phantom experiments with a custom-made motion phantom, and in-vivo healthy volunteer experiment without heating.
Introduction
MR-guided ultrasound thermal therapy has been used to treat tumors by direct ablation (>60 °C) or as a complementary treatment by sensitizing tissue to radiotherapy/chemotherapy (hyperthermia, 40-45 °C)1,2. Proton resonance frequency shift (PRFS)3 based MR thermometry, which relies on a voxel-wise analysis of phase difference between measured gradient echo (GRE) images, provides fast and accurate temperature monitoring for clinical MR-guided ultrasound thermal therapy. However, for treatment targets in abdominal organs, respiratory motion results in significant errors in the PRFS measurements4. This is especially problematic for hyperthermia therapy, which requires more accurate temperature measurements to ensure heating in a narrow temperature window (37-45 °C). Real-time volumetric MR thermometry is desirable for monitoring of temperature change in the entire region of treatment but is difficult to achieve due to long acquisition times for each frame. In particular, motion-robust, multi-slice, real-time MR thermometry is essential to meet the spatial and temporal requirements for ultrasound thermal therapy on abdominal or pelvic organs. In this study, we developed and evaluated a real-time volumetric MR thermometry acquisition and reconstruction pipeline for the temperature monitoring in abdominal organs. We quantified the temperature stability of this technique in simulation, motion phantom, and healthy volunteer experiments without heating.Methods
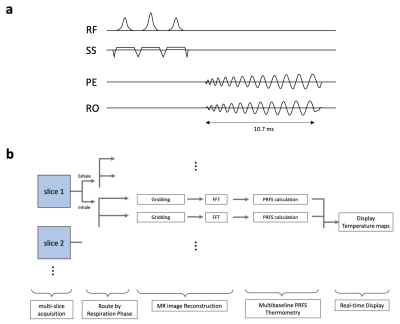
The accelerated multi-slice MR PRFS thermometry sequence was implemented on the RTHawk Research Platform (HeartVista, Menlo Park, CA)5. It featured an accelerated GRE sequence with a spiral readout trajectory (spiral readout length = 5.3 ms, number of interleaves=11) to achieve real-time volumetric imaging, and a multi-baseline6,7 reconstruction pipeline for the correction of motion artifacts in PRFS measurements (Fig.1). Respiratory motion was tracked with an integrated pencil-beam navigator sequence, interleaved with the thermometry sequence, and quantified with a temporal window of 17.3 ms. The acquired MR thermometry data was routed through the multi-branch PRFS reconstruction pipeline (15-20 branches), based on direction (exhale/inhale) and tissue displacement offset. Within each branch, image reconstruction and phase subtraction were performed on the data received within a specific portion of the respiratory cycle. The temperature maps generated by all branches were combined and continuously displayed on the console (Fig.1b). We evaluated the temperature stability of the technique with the following experimental protocols: 1) We implemented a simulation using a virtual phantom with respiratory motion integrated within the RTHawk Platform. 2) A low-cost MR compatible respiratory motion simulator was developed (Fig.2) and implemented for non-heating experiments with a tissue-mimicking phantom (InSightec, Israel) in a 3T MRI scanner (GE Healthcare, Waukesha, WI). Outside the MRI suite, a DC motor of 10-12 rpm was connected to a motion actuator, which increased or decreased the pressure inside the bag valve mask (BVM). The BVM was coupled via Tygon tubing to the compressible bag in the motion simulator in the MRI scanner. Various levels of inflation or deflation coupled with the compressible bag caused the cyclical motion of the phantom (~7.5 mm along the z-axis) to simulate breathing in the MRI scanner. 3) In-vivo healthy volunteer experiment (no heating) was conducted to evaluate the technique with physiological motion. The PRFS images were obtained in the axial plane of the liver. Single- and multi-baseline approaches were compared to evaluate the performance of the technique within all experiments. The MRI parameters for the experiments were used as the followings: TR/TE = 26/14.78 ms, FOV = 500 (simulation), 240 (phantom), 460 (volunteer) mm2 (resolution of 3.3, 1.52, 2.9 mm2, respectively), slice thickness = 5 mm, Number of branches = 20 (simulation, phantom) and 15 (volunteer). The acquisition time was around 303 ms per slice at TE=14.78 ms.Results
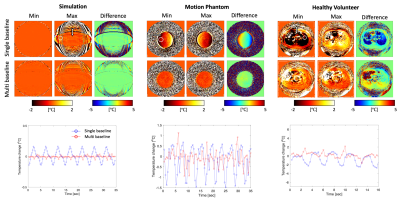
Figure 3 (top) shows PRFS temperature maps in simulation, phantom, and healthy volunteer experiments with single- and multi-baseline approaches at two positions of the respiratory cycle. The motion artifacts were clearly observed in the PRFS maps from single-baseline reconstruction, while the multi-baseline PRFS maps showed mitigation and reduction of the artifacts. Figure 3 (bottom) shows the mean of temperature within the ROI shown as a white circle across time for single- and multi-baseline PRFS acquisitions. The measured average temperature change was -0.01±0.02 °C (simulation)/-1.48±0.17 °C (phantom)/-0.67±0.8 °C (volunteer) in the multi-baseline PRFS acquisitions, compared to 0.06±0.10 °C (simulation)/-0.03±0.3 °C (experiment)/0.16±1.3 °C (volunteer) in the single baseline PRFS acquisitions. Single baseline acquisition showed periodic thermometry artifacts in temperature measurements, while multi-baseline reconstruction resulted in more homogeneous temperature maps with less artifacts throughout the breathing cycle.Discussion
Motion-robust, multi-slice, and real-time MR thermometry method developed herein demonstrated stable temperature measurements in the phantom with simulated respiratory motion and within a healthy volunteer experiment with free breathing. Compared to a conventional GRE sequence with cartesian readout, the spiral MR sequence did not show any geometric distortion or shift due to the low bandwidth of the spiral readout, while supporting frame rates up to 5 fps. Although the field drift correction was not applied in this study, we did not observe any temperature errors due to the main magnetic field drifts in our MRI system.Conclusion
This preliminary study indicates the all-in-one PRFS MR thermometry method has the potential to provide for real-time multi-slice temperature monitoring during free breathing with applications for hyperthermia or thermal ablation.Acknowledgements
This study was supported by NIH R21EB026018, R01EB025990, and R21CA230120.References
1. Diederich CJ. Thermal ablation and high-temperature thermal therapy: Overview of technology and clinical implementation. Int J Hyperthermia. 2005;21(8):745-753. doi:10.1080/02656730500271692
2. Schlesinger D, Benedict S, Diederich C, Gedroyc W, Klibanov A, Larner J. MR-guided focused ultrasound surgery, present and future. Med Phys. 2013;40(8). doi:10.1118/1.4811136
3. Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814-823. doi:10.1002/mrm.1910340606
4. Senneville BD de, Roujol S, Moonen C, Ries M. Motion correction in MR thermometry of abdominal organs: A comparison of the referenceless vs. the multibaseline approach. Magn Reson Med. 2010;64(5):1373-1381. doi:10.1002/mrm.22514
5. Ozhinsky E, Salgaonkar VA, Diederich CJ, Rieke V. MR thermometry-guided ultrasound hyperthermia of user-defined regions using the ExAblate prostate ablation array. J Ther Ultrasound. 2018;6. doi:10.1186/s40349-018-0115-5
6. Grissom WA, Rieke V, Holbrook AB, et al. Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs. Med Phys. 2010;37(9):5014-5026. doi:10.1118/1.3475943
7. Pichardo S, Köhler M, Lee J, Hynnyen K. In vivo optimisation study for multi-baseline MR-based thermometry in the context of hyperthermia using MR-guided high intensity focused ultrasound for head and neck applications. Int J Hyperthermia. 2014;30(8):579-592. doi:10.3109/02656736.2014.981299
Figures