0248
Fast MR thermometry based on propeller echo‐planar time‐resolved imaging with dynamic encoding (PEPTIDE)1Department of Engineering Physics, Tsinghua University, Beijing, China, 2Department of Radiology, School of Medicine, Stanford University, Stanford, CA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 5Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 6Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 7Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Echo‐planar time‐resolved imaging (EPTI) is a multi-shot EPI technique capable of rapidly obtaining distortion‐free and blurring‐free time-resolved multi-contrast images across the EPI readout. PROPELLER is an extension to EPTI, which incorporates PROPRELLER-like acquisition, to enable shot-to-shot motion toleration. In this work, PEPTIDE was applied to the MR thermometry application, where an image reconstruction framework that leveraged sparsity across blades of PEPTIDE rawdata was proposed. The potential in using PEPTIDE to provide distortion- and blurring-free temperature mapping at high temporal resolution was then demonstrated via simulated human brain PEPTIDE data with various temperature profiles.
Introduction
MR thermometry methods are essential for monitoring thermal distribution and deposition in real time during thermal ablation, requiring rapid acquisition and reconstruction. While fast acquisition pulse sequence EPI results in low-resolution and blurred images as a result of T2* decay and B0 inhomogeneity, echo planar time-resolved imaging (EPTI), a novel multi-shot EPI technology, allows extremely fast acquisition of distortion-free and blurring-free multi-contrast imaging and quantitative mapping1. EPTI has been proven feasible to apply to MR thermometry2.By incorporating k‐space encoding rotations into the EPTI sampling strategy, propeller echo‐planar time‐resolved imaging with dynamic encoding (PEPTIDE) repeatedly samples the low‐resolution k‐space center3. It is therefore capable of performing dynamic quantitative mapping such as temperature mapping with every single blade of PEPTIDE, and thus able to improve temporal resolution compared with quantitative mapping base on EPTI. Here we propose a new method for PEPTIDE fast image reconstruction through utilizing the correlation across the blades by temporal total variation, which has become a popular and powerful tool for image restoration and enhancement4. We apply the proposed method to temperature mapping where temperature develops rapidly and requires high temporal resolution. This provides a potential temperature monitoring approach for tumor hyperthermia applications. Among a number of temperature-sensitive MR parameters, proton resonance frequency shift (PRFS) is a preferred choice for many applications because of the excellent linearity and temperature dependence as well as its near independence of tissue type5. In the proposed method, temperature mapping is generated from complex reconstructed images by PRFS.
Method
Retrospective undersampling was performed to simulate PEPTIDE acquired data. As described in Fair et al,3 the gold standard image time-series and coil sensitivity maps were acquired at 1.0 × 1.0 × 3.0 mm2 resolution, 250 × 250 × 105 mm3 FOV with TRwhole-brain = 4 seconds in a healthy volunteer using a 32‐channel coil.Gaussian heat sources were added to the images to simulate the increasing temperatures with the following parameters: TE range of gradient echo = 1 - 36ms, number of TEs = 36, and echo spacing = 1 ms. Different profiles of heat distribution and deposition were simulated, depending on tissue property and heating speed.
With these inputs as a starting point, the data were undersampled in PEPTIDE sampling schemes using 36 golden-angle distributed ky-segments with RSEG = 36 and RPE = 4, while the subject remained stationary and no motion correction was applied to the PEPTIDE data. Within each blade, phase-encoding temporal GRAPPA was performed to reconstruct the unsampled points. The proposed reconstruction then solves for a TR(blade)-series of images that is sparse after temporal total variation along the TR dimension:
\[argmin_x||PSFx-y||^2_2+\lambda||TV(x)||_1\]
where $$$P$$$ is the sampling operator for full-sampled blades, $$$F$$$ is the Fourier transform, $$$S$$$ is the multiplication with the sensitivities, $$$y$$$ is the complex subtracted k-space data, $$$TV(x)$$$ is the total variation (TV) regularization of PEPTIDE data along the TR dimension.
Signals acquired from different echoes in each blade were used to calculate the mean temperature changes under diverse TEs using PRFS. The reconstruction pipeline is shown in Figure 1.
For validation, the reconstructed magnitude PEPTIDE images were compared against the fully sampled reference as well as those based on EPTI sampling schemes. Temperature curves were derived from the proposed PEPTIDE-mappings and compared to the reference temperature curve.
Results and Discussion
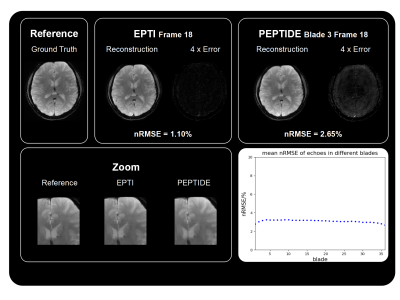
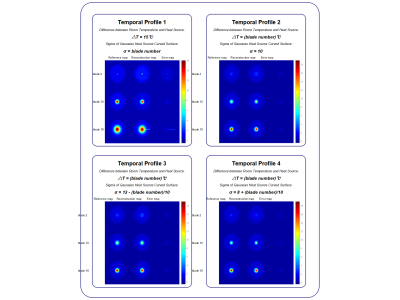
Figure 2 shows the reconstructed magnitude images of the simulation and those reconstructed from EPTI sampling schemes with the same Rseg. Images with high spatial resolutions were reconstructed through the proposed reconstruction method for each blade. The corresponding 4x intensity error maps are displayed for both EPTI and PEPTIDE, showing a small increase in reconstruction errors for PEPTIDE, mainly located outside the brain center. Note that due to the temporal total variation there is slight smoothing in the reconstructed images from PEPTIDE. Images of various blades of PEPTIDE demonstrate low and similar normalized root mean square error (nRMSE).Figure 3 shows the temperature mappings of 3 out of the 36 blades. For all of the four temporal profiles, this method resulted in high-quality reconstruction results of temperature mapping where there is no considerable difference in the regions of interest.
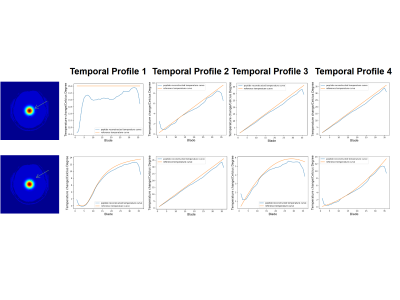
Figure 4 shows the temperature curves plotted against time (TR). Two typical voxels were selected to demonstrate the change of temperature in comparison with the room temperature. The reconstructed curves are consistent with the reference for most of the TRs, while in the edge there are some mismatches due to the lack of sufficient neighboring blades during temporal total variation reconstruction.
Conclusion
We have presented an improved dynamic reconstruction technique for high spatio-temporal PEPTIDE images compared with conventional EPTI. We then applied it to temperature mapping and proved the theoretical feasibility of PEPTIDE thermometry. PEPTIDE has the advantage over EPTI in thermometry for improved temporal resolution by reconstructing temperature mapping of every single TR. Future work will focus on phantom and in vivo validations.Acknowledgements
No acknowledgement found.References
1. Wang, Fuyixue, et al. "Echo planar time‐resolved imaging (EPTI)." Magnetic resonance in medicine 81.6 (2019): 3599-3615.
2. Wending Tang, et al. "MR thermometry based on PRF using echo planar time-resolved imaging (EPTI)." In: Proceedings 28th Scientifific Meeting, ISMRM. Paris, 2020.
3. Fair, Merlin J, et al. "Propeller Echo‐planar Time‐resolved Imaging with Dynamic Encoding (PEPTIDE)." Magnetic Resonance in Medicine 83, no. 6 (2019): 2124-137.
4. Landi, Germana, Piccolomini, Elena Loli, and Zama, Fabiana. "A Total Variation-Based Reconstruction Method for Dynamic MRI." Computational and Mathematical Methods in Medicine 9, no. 1 (2008): 69-80.
5. Rieke, Viola, and Butts Pauly, Kim. "MR Thermometry." Journal of Magnetic Resonance Imaging 27, no. 2 (2008): 376-90.
Figures