0247
Susceptibility artifact correction in MR thermometry for monitoring of mild RF hyperthermia using total field inversion1Technical University of Munich, Munich, Germany, 2Department of Medicine III, University Hospital, LMU Munich, Munich, Germany, 3Erasmus MC Cancer Institute, Rotterdam, Netherlands, 4Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany
Synopsis
Motion-induced susceptibility changes induce field variations, leading to large errors during MR thermometry based on the linear proton resonance frequency shift. These artefacts aggravate temperature quantification in the face of both the long treatment duration and the mild temperature change during mild RF hyperthermia treatments. We show with the help of simulations, a phantom heating experiment, volunteer scans and mild hyperthermia treatment of a patient with cervical cancer and a sarcoma patient how to correct for this artefact source by methods known from quantitative susceptibility mapping. The recently introduced total field inversion shows advantages over the background field removal methods.
Purpose
Mild heating of various cancer types sensitizes tumors to radio- and chemotherapy1. This treatment modality has thus found its way into clinical practice in recent years, including the treatment of sarcoma patients and patients with cervical cancer2-5. MR temperature monitoring of mild hyperthermia (HT) treatment (40-44°C) of cancer is done by exploiting the linear resonance frequency of water6-8. However, susceptibility distribution changes between different time points, due to digestive motion including gas or movement of air bubbles inside the circulating water bolus create much stronger phase changes than the temperature-induced phase change of -0.01ppm/°C9. Recently, it was proposed to correct for the susceptibility artifacts by solving the Laplacian boundary value problem (LBV)10 or by projection onto dipole (PDF)11,12. These two methods are well-known in the context of quantitative susceptibility mapping for separating the foreground from the background fields. However, PDF is known to overfit especially at air-tissue interfaces and Laplacian-based method reduce the field of view. Total field inversion (TFI)13 has been proposed to alleviate the above problems and is presently applied.Theory
To estimate the phase contributions originating from susceptibility sources the following TFI cost function is solved similar to13:$$y'=\underset{y}{\arg\min}=\frac{1}{2}||w(f-d*Py)||_1+\lambda{||}\nabla{P}y||_1,$$where$$$\:{w}\:$$$the a data weighting term,$$$\:{f}\:$$$is the input phase,$$$\:{d}\:$$$is the dipole kerne in$$$\:{k}\:$$$-space,$$$\:{P}\:$$$the preconditioner and$$$\:\nabla\:$$$the gradient operation. The final susceptibility distribution is computed as$$$\:\chi=Py$$$.Methods
The phantom heating experiment as well as the volunteer data set were acquired on a 1.5T GE system (GE Discovery MR450w/USA). The BSD2000-3D Sigma Eye MR-compatible RF applicator (PYREXAR Medical/USA) consists of 24 dipole antennas arranged in three rings of 8 antennas. No water was circulated during the phantom measurement or the volunteer study. For phase unwrapping, we used the code available at (https://gitlab.com/veronique_fortier/Quality_guided_unwrapping).Simulations: The simulated matrix size was$$$\:150\times150\times150$$$, which corresponded to a FOV of$$$\:50\times50\times50cm^3$$$. The simulated field disturbance originated from a susceptibility change $$$\Delta\chi\:$$$within a sphere of$$$\:2\:$$$cm diameter in the center. The susceptibility difference $$$\Delta\chi$$$ was the one between water and air. A 3D Gaussian temperature increase profile with a peak value of 10°C and a standard deviation of$$$\:5\:$$$pixels was added to the image. A spatially variant 1st order phase was added to imitate B0 drift.
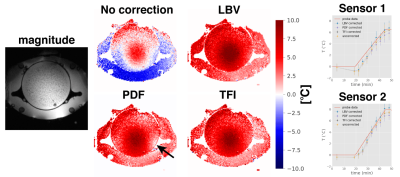
Phantom heating: A double echo GRE with slice interleaved acquisition scheme was scanned for temperature monitoring. Using the phase signal at both TEs compensated for conductivity change-induced phase offsets (7) (TR=620$$$\:$$$ms, 25 slices, scan time=83 s, TE_1=4.8 ms, TE_2=19.1 ms, matrix size=$$$128\times128$$$, FOV=50 cm$$$\times\:$$$50 cm, flip angle=40°, slice thickness=10 mm, bandwidth=325.5 Hz/px.)
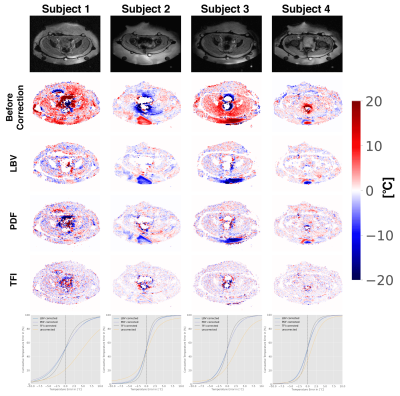
Volunteers: Single echo data sets were acquired for in total 4 volunteer data sets (3 male, 1 female) (TE/TR=15ms/21ms, 20 slices matrix size=$$$128\times160$$$, FOV=50 cm$$$\times\:$$$50 cm, flip angle=14°, slice thickness=10mm). As$$$\:$$$at constant temperature, the conductivity bias did not need to be considered, a single echo$$$\:$$$acquisition scheme was$$$\:$$$sufficient. 30 min passed between the two acquisition time points.
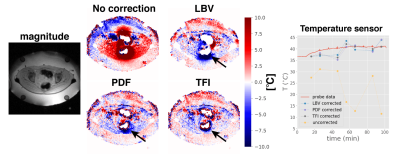
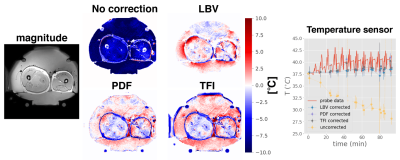
Patient treatment: Patient treatment$$$\:$$$scans were performed with the$$$\:$$$approval of the respective local ethics board. A Double-Echo Gradient Echo (DEGRE) acquisition corrected for the conductivity bias14. We evaluated the mild RF-HT of a cervical tumor treated$$$\:$$$with the aforementioned BSD2000-3D Sigma Eye applicator inside a$$$\:$$$1.5T GE system. Furthermore, we evaluated the mild RF-HT of a sarcoma in the thigh, which was treated with the more novel BSD-2000 3D/MR applicator inside a 1.5T Philips system.
Results
Simulations: All three methods can eliminate both the linear phase as well as the dipole, while preserving the simulated heat distribution. While the LBV method performed best, PDF and TFI performed similarly, as seen in$$$\:$$$the cumulative error plot(Fig.1).Phantom$$$\:$$$heating: The PDF algorithm overestimates the background field effect particularly at the border of the phantom(black arrow in Fig.2). Furthermore, we could see that all three$$$\:$$$methods successfully removed the$$$\:$$$B0 drift effect and matched well to the sensor probe data.
Volunteers: In contrast to the simulation results, here, TFI showed the best performance in terms of$$$\:$$$cumulative error in all 4 volunteers(Fig.3).
Patient treatment: The advantage of preserving pixel layers becomes particularly apparent in case of tumors in proximity to tissue/air interfaces, as$$$\:$$$for the case$$$\:$$$of cervical cancer treatments with MR thermometry monitoring(Fig.4). It is important to note that the severity and spatial extension of the susceptibility artifacts would not allow for B0 drift correction, as the silicon tubes would be superimposed by that same artifact. Results for a sarcoma patient treatment are shown(Fig.5).
Discussion
TFI performs more robustly in the presence of noise, as seen in the phantom and volunteer measurements. This is a big advantagein the context of RF-HT treatments, as the heating device precludes the use of other MR receive coils than the body coil and thus suffers from low SNR. In contrast to LBV, all pixels are preserved. This is particularly useful as the tumor is oftentimes located next to intestinal gas, and thus at the edge of the foreground mask.Conclusion
LBV, PDF, and TFI successfully remove susceptibility artifacts without subtracting the phase change due to temperature change. The B0 drift correction comes for free. The PDF algorithm has the tendency to overestimate the background field contribution, whereas the LBV method peels valuable pixel layers away for fitting. Thus, TFI might be a promising method for gaining accurate temperature maps during mild RF-HT treatments.Acknowledgements
No acknowledgement found.References
1. Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia. 2006;22(3):191-6.
2. Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018;4(4):483-92.
3. Issels R, Kampmann E, Kanaar R, Lindner LH. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. Int J Hyperthermia. 2016;32(1):89-95.
4. Bucklein V, Limmroth C, Kampmann E, Schuebbe G, Issels R, Roeder F, et al. Ifosfamide, Carboplatin, and Etoposide (ICE) in Combination with Regional Hyperthermia as Salvage Therapy in Patients with Locally Advanced Nonmetastatic and Metastatic Soft-Tissue Sarcoma. Sarcoma. 2020;2020:6901678.
5. Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, et al. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer. 2009;45(11):1969-78.
6. Rieke V, Vigen KK, Sommer G, Daniel BL, Pauly JM, Butts K. Referenceless PRF shift thermometry. Magn Reson Med. 2004;51(6):1223-31.
7. Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: correlation with response and direct thermometry. Cancer. 2006;107(6):1373-82.
8. Gellermann J, Wlodarczyk W, Hildebrandt B, Ganter H, Nicolau A, Rau B, et al. Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res. 2005;65(13):5872-80.
9. Winter L, Oberacker E, Paul K, Ji Y, Oezerdem C, Ghadjar P, et al. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int J Hyperthermia. 2016;32(1):63-75.
10. Zhou D, Liu T, Spincemaille P, Wang Y. Background field removal by solving the Laplacian boundary value problem. NMR Biomed. 2014;27(3):312-9.
11. Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24(9):1129-36.
12. Wu M, Mulder HT, Baron P, Coello E, Menzel MI, van Rhoon GC, et al. Correction of motion-induced susceptibility artifacts and B0 drift during proton resonance frequency shift-based MR thermometry in the pelvis with background field removal methods. Magn Reson Med. 2020;84(5):2495-511.
13. Liu Z, Kee Y, Zhou D, Wang Y, Spince-maille P. Preconditioned Total Field Inver-sion (tfi) Method for Quantitative Susceptibil-ity Mapping. Magnetic Resonance in Medicine2016; 78:303–315. 10.1002/mrm.26331.
14. Peters RD, Henkelman RM. Proton-resonance frequency shift MR thermometry is affected by changes in the electrical conductivity of tissue. Magn Reson Med. 2000;43(1):62-71.
Figures