0243
Detection of ionic bonding using IMMOBILISE MRI and theoretical description of the relayed NOE transfer mechanism1Institute of Biomedical and Health Engineering, Shenzhen Institutes of Advanced Technology, Shenzhen, China, 2The Russell H. Morgan Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Information Science and Technology, Northwest University, Xian, China
Synopsis
Saturation transfer MRI has previously been applied to study transient molecular binding using the IMMOBILISE MRI approach, yet the multiple mechanisms of magnetization transfer during such binding are still under investigation. We studied electrostatic-interaction-mediated molecular binding of small substrates (choline and arginine) to ion-exchange column media. A theoretical model using relayed NOEs to exchangeable protons in the bound substrate is proposed to quantitatively describe the saturation transfer during such binding.
Introduction
Electrostatic interactions play an essential role in many biological binding processes.1 It has been previously shown that transient molecular binding can be detected with MRI using the IMMOBILISE (for “IMaging of MOlecular BInding using Ligand Immobilization and Saturation Exchange”) experiment.2,3 Here we show it is possible to detect transient binding of small substrate molecules (choline and arginine) via electrostatic interactions to ion-exchange media. An exchange-relayed NOE (rNOE) based model (Fig. 1) is proposed to quantitatively describe the magnetization transfer from the aliphatic protons of the bound substrate to water.Materials and Methods
All ion-exchange media (with functional groups such as -SO3-, -COO-, from Bio-Rad Laboratories, Inc., Hercules, CA) were washed with 2M NaCl and then phosphate-buffered saline (PBS). Arginine and choline were dissolved separately in PBS, and then mixed with the ion-exchange medium. The mixture was pH-adjusted.CEST MRI experiments were performed on a 17.6T Bruker Avance III (Bruker Biospin, Ettlingen, Germany) at 20°C using a 4s CW saturation pulse followed by RARE MRI readout. The irradiation frequency was stepped over the proton spectral range (±6 ppm in steps of 0.1 ppm) and the B1 power was varied from 0.3 to 2 $$$\mu$$$T. Z-spectral shifts caused by B0 inhomogeneities were corrected using the WASSR method.4 Z-spectral components were quantified using multi-Lorentzian fitting, as described previously.5
Results
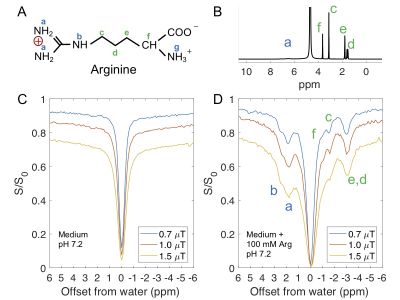
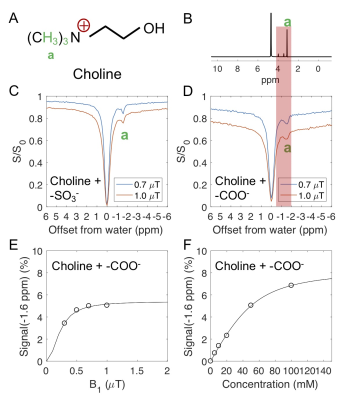
Ionic resin particles contain charged groups and are routinely used in ion-exchange chromatography. Figs. 2D shows the Z-spectra of arginine solution mixed with ionic resins (particle size: ~50 $$$\mu$$$m, with the function group -SO3-). At a pH of 7.2, two peaks centered at +1.8 ppm and +2.5 ppm, corresponding to the guanidino group (position a, as shown in Fig. 2A) and amide group (position b) of arginine, respectively suggest these protons are in slow exchange on the NMR time scale at this field strength.6 On the negative side of the Z-spectra, three peaks at -0.9, -1.7, -3.1 ppm are present, showing the rNOE based magnetization transfer from arginine aliphatic protons7 to water.Similarly, choline contains a positive charged head, (Fig. 3A) which interacts with negative functional groups (-SO3- and -COO-) in the matrix. This allows enhanced detection of choline aliphatic protons at -1.6 ppm of the Z-spectra. (Fig. 3C, D) The rNOE signal dependence on total choline concentration was not linear. (Fig. 3F)
The following model is proposed to describe the magnetization transfer process from ligand aliphatic protons to water during the binding process,
$$\begin{array}{c}\mathrm{k}_{\mathrm{on}} \quad \sigma_{l e} \quad \mathrm{k}_{\mathrm{ew}} \\ \mathrm{L} \leftrightarrow \mathrm{LB} \leftrightarrow \mathrm{E} \leftrightarrow \mathrm{W} \\ \mathrm{k}_{\mathrm{off}} \quad \sigma_{e l} \quad \mathrm{k}_{\mathrm{we}}\end{array}$$
Where $$$k_{on}$$$ and $$$k_{off}$$$ are ligand (L) binding on/off rates, $$$k_{ew}$$$ and $$$k_{we}$$$ are chemical exchange rates between free water (W) and exchangeable protons (E), $$$\sigma_{le}$$$ and $$$\sigma_{el}$$$ are the effective cross relaxation rates between bound ligands (LB) aliphatic protons and exchangeable protons (E). In CEST experiments, when a long saturation pulse is applied on ligand aliphatic proton (L), the evolutions of the z-magnetizations for each pool can be described by a set of modified Bloch equations,
$$\begin{array}{l}\frac{dL_{z}}{dt}=-\rho_{L}\left(L_{z}-L_{z, 0}\right)-k_{on} L_{z}+k_{off} L B_{z}-w_{1} L_{y} \quad[1] \\ \frac{dLB_{z}}{dt}=-\rho_{LB}\left(LB_{z}-LB_{z, 0}\right)-\sigma_{el}\left(E_{z}-E_{z, 0}\right)+k_{on} L_{z}-k_{off} L B_{z} \quad[2]\\ \frac{dE_{z}}{dt}=-\rho_{E}\left(E_{z}-E_{z, 0}\right)-\sigma_{l e}\left(LB_{z}-LB_{z, 0}\right)-k_{ew} E_{z}+k_{we} W_{z}\quad[3]\\ \frac{d W_{z}}{dt}=-\rho_{W}\left(W_{z}-W_{z, 0}\right)+k_{ew} E_{z}-k_{we} W_{z}\quad[4]\end{array} $$
$$$\rho_{L}$$$, $$$\rho_{LB}$$$, $$$\rho_{E}$$$, $$$\rho_{W}$$$ are the longitudinal auto-relaxation rates for the four pools. $$$w_{1}$$$ is the B1 power. Notice that when the ligand is bound, the signals broaden and cannot be efficiently saturated, so no $$$w_{1}$$$ term is included. Assuming steady-state is reached, and $$$\frac{dLB_{z}}{dt}=0$$$, $$$\frac{dE_{z}}{dt}=0$$$, $$$\frac{dW_{z}}{dt}=0$$$ and at chemical exchange equilibrium $$$k_{on} L_{z, 0}-k_{off} LB_{z, 0}=0$$$, $$$k_{ew} E_{z, 0}-k_{we} W_{z, 0}=0$$$. We can obtain the analytical solution:
$$rNOE \equiv \frac{W_{z, 0}-W_{z}}{W_{z, 0}}=\alpha * e * f\quad[5]$$
Where $$$\alpha$$$ is saturation factor, $$$\alpha=\frac{\left(L_{z, 0}-L_{z}\right)}{L_{z, 0}}$$$, e is the enhancement factor, $$$f=\frac{L_{z, 0}}{W_{z, 0}}=\frac{[L]}{[W]}$$$,
$$e=\frac{k_{ew} \sigma_{le} k_{on}}{\sigma_{el} \sigma_{le}\left(\rho_{W}+k_{we}\right)-k_{off} \rho_{E} \rho_{W}-k_{off} k_{ew} \rho_{W}-k_{off} \rho_{E} k_{we}-\rho_{LB} \rho_{E} \rho_{W}-\rho_{LB} k_{ew} \rho_{W}-\rho_{LB} \rho_{E} k_{we}}\quad[6]$$
In the slow tumbling limit, we assume $$$-\sigma_{e l} \approx-\sigma_{l e} \approx \rho_{LB} \approx \rho_{E} \gg \rho_{W}$$$,
$$e \approx \frac{-k_{ew} \sigma_{le} k_{on}}{k_{off} \rho_{E} \rho_{W}+k_{off} k_{ew} \rho_{W}+k_{off} \rho_{E} k_{wr}+\rho_{L B} k_{ew} \rho_{W}} \approx \frac{1}{\left(\frac{\rho_{w}}{k_{ew}}+\frac{k_{we}}{k_{ew}}+\frac{\rho_{W}}{-\sigma_{le}}+\frac{\rho_{W}}{k_{off}}\right)} \frac{k_{on}}{k_{off}}\quad[7]$$
$$rNOE \approx \alpha * \frac{1}{\left(\frac{\rho_{W}}{k_{ew}}+\frac{k_{we}}{k_{ew}}+\frac{\rho_{W}}{-\sigma_{l e}}+\frac{\rho_{W}}{k_{off}}\right)} * \frac{[L] k_{on}}{[W] k_{off}}=\alpha * e^{\prime} * \frac{[LB]}{[W]}\quad[8]$$
It is useful to rewrite [LB] using known parameters: $$$E_T$$$, total receptor concentration, $$$L_T$$$, total ligand concentration, and $$$K_D$$$, the dissociation constant:8,9
$$[LB]=\frac{1}{2}\left(L_{T}+E_{T}+K_{D}\right)-\frac{1}{2} \sqrt{\left(L_{T}+E_{T}+K_{D}\right)^{2}-4 L_{T} E_{T}}\quad[9]$$.
From Eqs. 8 and 9, it can be seen that the detected rNOE signal in the binding system is dependent on the population of ligand aliphatic protons in the bound state ([LB]), which is a function of total ligand concentration ($$$L_T$$$, Fig. 3F) and the electrostatic interaction or the binding equilibrium (Eq. 9).8,9
Discussion&Conclusion
The results from arginine and choline show the molecule can bind to different negatively charged groups via non-specific electrostatic interactions, and the rNOE signal depends on the total choline concentration non-linearly, which agrees with the model (Eq. 9).This proof-of-concept study suggests that CEST MRI could have the potential to probe electrostatic interactions in vivo. The proposed model is useful for understanding the basis of CEST contrast in situations involving molecular binding.
Acknowledgements
No acknowledgement found.References
1 Nakamura, H. Roles of electrostatic interaction in proteins. Quarterly Reviews of Biophysics 29, 1-90, (1996).
2 Yadav, N. N. et al. Detection of dynamic substrate binding using MRI. Scientific reports 7, 10138 (2017).
3 Lenich, T., Pampel, A., Mildner, T. & Möller, H. E. A new approach to Z-spectrum acquisition: prospective baseline enhancement (PROBE) for CEST/Nuclear Overhauser Effect. Magnetic Resonance in Medicine 81, 2315-2329, (2019).
4 Kim, M., Gillen, J., Landman, B. A., Zhou, J. & Van Zijl, P. C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magnetic Resonance in Medicine 61, 1441-1450 (2009).
5 Zhou, Y., van Zijl, P. C., Xu, J. & Yadav, N. N. Mechanism and quantitative assessment of saturation transfer for water‐based detection of the aliphatic protons in carbohydrate polymers. Magnetic Resonance in Medicine, (2020).
6 Klavan, L. & Crothers, D. M. High‐resolution NMR of exchangeable protons in arginine, oligoarginines, and the arginine‐rich histone tetramer. Biopolymers: Original Research on Biomolecules 18, 1029-1044 (1979).
7 Wishart, D. S. et al. HMDB: a knowledgebase for the human metabolome. Nucleic acids research 37, D603-D610 (2009).
8 Lepre, C. A., Moore, J. M. & Peng, J. W. Theory and Applications of NMR-Based Screening in Pharmaceutical Research. Chemical Reviews 104, 3641-3676, (2004).
9 Gossert, A. D. & Jahnke, W. NMR in drug discovery: A practical guide to identification and validation of ligands interacting with biological macromolecules. Progress in nuclear magnetic resonance spectroscopy 97, 82-125 (2016).
Figures