0240
Optimized simultaneous 3D proton MRF and sodium MRI1Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Vilcek Institute of Graduate Biomedical Sciences, NYU Langone Health, New York, NY, United States, 3Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 4ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia
Synopsis
In this work, we present an optimized 3D technique that can simultaneously acquire quantitative 1H density, T1, T2, B1+ maps and a 23Na image of the whole head in a reasonable scan time (~20 min).
Introduction
Sodium (23Na) MRI can reveal valuable metabolic information1. However, due to their low resolution, 23Na images are usually paired with high resolution proton (1H) MRI to reveal the underlying morphology. Recently, a technique to simultaneously acquire sodium images during a proton magnetic resonance fingerprinting (MRF) scan was demonstrated2, which can ease the burden of sequential 23Na and 1H scans. This method simultaneously acquires quantitative 1H density (PD), T1, T2, B1+ maps and a 23Na density image, all in 3D with a 20 min total scan time3. In this work, we focus on the optimization of the 3D acquisition technique to obtain parametric maps with reduced artifacts.Methods
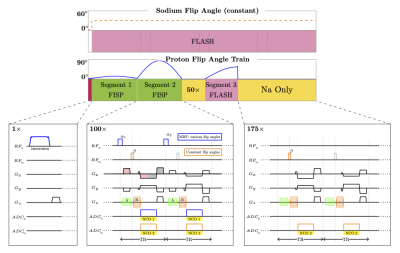
Our sequence uses a stack-of-stars sampling scheme4. Each TR (7.5ms), a none-selective 1H and a non-selective 23Na pulses are applied, followed by one simultaneous readout for both nuclei (Fig.1). We strategically divide the partition gradient moment between the 1H pulse and 23Na pulse, such that images from both nuclei will have the same slice thickness3. Similarly, the frequency encoding gradient moments are distributed such that we can acquire a full radial trajectory for 1H while also obtain a center-out readout for 23Na, which allows for a factor ~2 between the 23Na and 1H resolutions, instead of the factor ~4 (γNa/ γH) as for the 2D version.The previous 3D implementation inherited the flip angle train from the 2D implementation, which is sub-optimal for fast 3D imaging without full relaxation between repetitions. Therefore, we empirically redesigned the flip-angle train with a short delay in mind. The new train starts with an adiabatic inversion, followed by 275 excitations of variable flip angles (Fig1), instead of 1000 excitations from the original sequence3. The first segment (TR 1-100) includes flips angles up to 20° providing signal to readout the T1 recovery while contributing minimal saturation. The second segment (TR 101-200) contains higher flip-angles (up to 90°) to help encode T2. A 50-TR delay is inserted to recover some magnetization before the 3rd segment (TR 251 ~ 325), which is RF spoiled to help encode B1+, with flip-angle up to 60°. A short delay (TR 326 ~ 500) is also added before the next inversion.
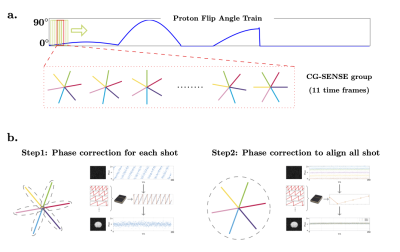
On the sodium side, a constant flip angle (30°) is applied at every other TR to reduce SAR and obtain a larger effective gradient spoiling moment for sodium (TR for sodium is thus 15 ms). The sodium acquisition continues throughout the whole scan without interruption (see Fig. 1).For the proton MRF reconstruction, CG-SENSE is applied to eliminate the radial artifacts5. After removing all the delays, radial samples from 275 TRs are reconstructed with a sliding window covering 11 TRs (Fig. 2a). The MRF dictionary is also compressed/averaged with the same sliding window along the time domain.
To enable the simultaneous acquisition, the received chain was modified as described in Meyerspeer et al6. Phase correction for the sodium signal is necessary due to the asynchronization between the MR system and the external frequency generator. As shown in Figure 2b, there is a linear phase drift between the samples along each shot. In addition, for all shots in the same partition, another layer of phase correction is in need to align all shots. A dictionary matching scheme is applied to correct these phase drifts3.
All experiments were performed at 7T (MAGNETOM, Siemens, Erlangen, Germany) using a 16-channel-Tx/Rx dual-tuned coil developed in-house7. All images were reconstructed in MATLAB (Mathworks, Natick, MA, USA) using the CG-SENSE5 and Fessler NUFFT toolbox8.
A seven-tubes cylindrical phantom (1H T1: 600~1600ms, T2: 40~180ms) was used to verify the accuracy of the multiparametric 1H maps, using the previous 3D sequence as a reference. In addition, one healthy volunteer (39yo male) was scanned in accordance with our IRB protocol. The 3D sequence parameters for phantom and invivo experiments were: 1H 160×160 / 23Na 84×84 matrix, 1H 1.5×1.5 mm2 / 23Na 3.0×3.0 mm2 in-plane resolution, 3.0 mm slice thickness, 56 slabs, 6 shots per slab, total scan time 21 min. In this case, the sodium 3D data has isotropic resolution.
Results & Discussion
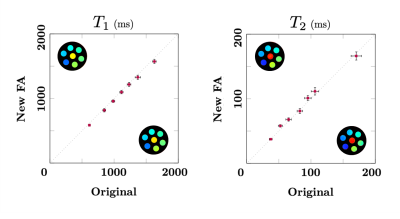
The T1 and T2 values in phantoms obtained with the new flip-angle train show an excellent correlation with the reference sequence (Fig. 3). The 3D invivo results are shown in Fig. 4, where sodium and proton data were acquired simultaneously in one perfectly co-registered scan. After the phase drift correction, clean sodium images were obtained. For proton MRF, the shorter train length and delay enabled us to acquire more radial samples per time-frame without extending the scan time, and thus reduce radial aliasing artifacts (under-sampling factor per time frame is reduced). Combined with the sliding window CG-SENSE reconstruction, residual streak-like artifacts in the T1 and T2 maps were significantly reduced.Conclusion
The optimized 3D implementation provides the opportunity to simultaneously obtain high-quality volumetric 1H MRF and 23Na MRI of the brain. We hope that this technique will make sodium MRI more applicable for future clinical research, as it can now be acquired during the 1H scan without extending the MRI protocol.Acknowledgements
The research reported in this publication was supported by the NIH/NIBIB grant R01 EB026456, and performed under the rubric of the Center for Advanced Imaging Innovation and Research, a NIBIB Biomedical Technology Resource Center (P41 EB017183).References
1. Madelin G, & Regatte R R. Biomedical applications of sodium MRI in vivo. Journal of Magnetic Resonance Imaging, 2013;38:511-529.
2. Yu, Z., Madelin, G., Sodickson, D. K., & Cloos, M. A. Simultaneous proton magnetic resonance fingerprinting and sodium MRI. Magnetic resonance in medicine,2020, 83(6), 2232-2242.
3. Yu, Z., Hodono, S., Wang, B., Dergahyoval, O., Zhang, B., Brown, R., Sodickson, D. K., Madelin, G., & Cloos, M. A. Simultaneous 3D proton MRF and sodium MRI. 2020 ISMRM&SMRT virtual conference & exhibition
4. Block KT, Chandarana H, Milla S, et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J Korean Soc Magn Reson Med. 2014;18(2):87-106. doi:10.13104/jksmrm.2014.18.2.
5. Pruessmann, K. P., Weiger, M., Börnert, P., & Boesiger, P. Advances in sensitivity encoding with arbitrary k‐space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2001, 46(4), 638-651.
6. Meyerspeer M, Magill A W, Kuehne A, Gruetter R, Moser E, & Schmid A I. Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magnetic resonance in medicine, 2016;76:1636-1641.
7. Bili Wang, Martijn Cloos, Ryan Brown, Guillaume Madelin, Daniel K. Sodickson, Bei Zhang. Dual-Tuned 16-Channel TxRx Array for Sodium & Proton Brain Imaging at 7T. 2019.
8. Fessler J A, & Sutton B P. Nonuniform fast Fourier transforms using min-max interpolation, IEEE Transactions on Signal Processing, 2003;51:560-574.
Figures