0234
Clinical translation of simultaneous metabolic and perfusion imaging with hyperpolarized [1-13C]pyruvate and [13C, 15N2]urea1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Graduate Program in Bioengineering, UC Berkeley – UCSF, San Francisco, CA, United States, 3Medicine, University of California, San Francisco, San Francisco, CA, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Research, Niskayuna, NY, United States
Synopsis
Altered metabolism and perfusion are implicated in cancer’s underlying pathophysiology. Prior preclinical and clinical studies have shown that metabolic and perfusion imaging could provide a sensitive and specific evaluation of tumor grade and therapeutic response. We aim to develop a dual-agent hyperpolarized 13C MR technique for simultaneous metabolic and perfusion imaging in humans. Here, we report the technical developments towards its clinical translation: 1) formulation and co-polarization system of 13C pyruvate and urea, 2) imaging probe characterization, 3) safety-related studies, and 4) multi-probe imaging sequence. Upon FDA approval, this work would lead to the first-in-human dual-agent hyperpolarized MR study.
Background and Motivation
Hyperpolarized (HP) 13C pyruvate MR is under clinical investigation as a diagnostic tool for characterizing cancer aggressiveness and monitoring therapeutic response1. HP 13C MR has the unique capability of simultaneous multi-probe imaging, a key advantage compared to other molecular imaging techniques such as positron emission tomography (PET)2. Previous preclinical studies have shown that combining HP 13C pyruvate with a perfusion imaging agent – HP 13C urea – could provide a more sensitive evaluation of cardiac physiology3, tumor biology4,5, and response to therapy6,7 (Figure 1). Moreover, the addition of an extracellular perfusion agent could potentially improve kinetic modeling of pyruvate metabolism by providing an independent measurement of vascular input and tissue distribution.We aim to bring this dual-agent HP MRI to clinical research studies. Here, we present the technical developments towards the clinical translation of combined HP 13C pyruvate and urea MR for simultaneous metabolic and perfusion imaging.
Methods and Results
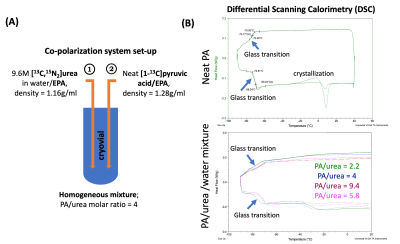
1. Formulation: In preclinical studies, pyruvic acid (PA) and urea are sequentially frozen with minimal or no contact2; this approach is incompatible with the clinical polarizer and fluid path designs. Additionally, urea requires a solvent and glassing agent such as glycerol, which is undesirable for human studies. With these considerations, urea was dissolved in water first, then mixed with neat PA, resulting in a homogeneous mixture contains approximately 10M [1-13C]PA, 2.5M [13C,15N2]urea, and 12.5mM GE trityl radical (Figure 2A). [13C,15N2]urea was used because of its prolonged T1 at low-field compared to [13C]urea, preserving polarization during sample transfer.2. Characterization: Reproducible glass formation is critical for dynamic nuclear polarization. Using differential scanning calorimetry (DSC), we found that PA/urea/water mixtures have similar glass transition temperatures (Tg) to neat PA (ranging from -70 to -80 °C), a desirable glass material, and did not observe crystallizations for a wide range of water content in the formulation (Figure 2B).
To test polarization performance, approximately 1.1ml combined 13C PA and urea probe was polarized on a 5T SPINlab polarizer using 139.97 GHz microwave for 3-4 hours, then rapidly dissolved in 41ml super-heated (130 °C), pressurized sterile water and subsequently neutralized using equivalent NaOH and Tris buffer. This procedure produced approximately 150mM sodium pyruvate, 35 mM urea, and < 3 µM EPA, with liquid-state polarization of 39.9 ± 5.9 % for pyruvate, 39.1 ± 7.3 % for urea (n = 20). A representative 13C NMR spectrum of HP dissolution acquired on a 1.4T bench-top spectrometer is shown in Figure 3A.
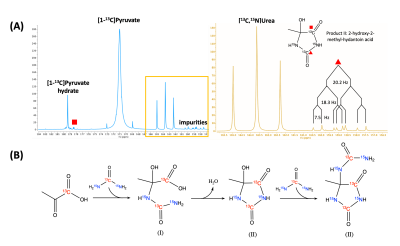
3. Safety-related studies for clinical translation: We investigated potential impurities in the PA-urea mixture by leveraging high-resolution NMR and HPLC-MS. Three impurity compounds were identified in hyperpolarized and mock dissolution studies (Figure 3B). The total molar % concentration of impurities is 2.13% in the final dissolution based on MS and 2.5% based on NMR.
We then investigated any potential toxicity of co-polarized pyruvate and urea and the impurities identified. Sprague Dawley rats were injected with normal saline (n = 3), co-polarized dissolution (n = 10), or isolated impurity products (n =10). We did not observe any adverse responses in vital signs (pulse, respiratory rate, oxygen saturation, temperature, body weight), laboratory tests (complete blood counts, liver-kidney panel), or gross pathology of major organs.
Finally, we developed a Standard Operating Procedure (SOP) to produce a sterile co-polarized 13C pyruvate and urea solutions for human injection, then validated the SOP by three consecutive successful Performance Qualification (PQ) trials. The SOP can produce sterile products with probe concentrations, pH, and polarizations comparable to the aforementioned characterizations (Figure 4).
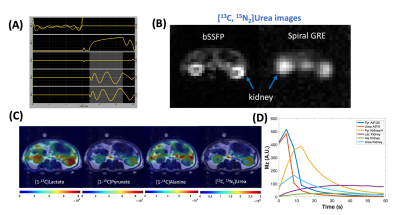
4. Metabolite-specific imaging for co-polarized pyruvate and urea: The split peaks of [13C,15N2]urea (JCN = 20Hz, Figure 2A) causes off-resonance artifacts when long readout trajectories were used such as Echo Planar Imaging (EPI) or spiral gradient echo (GRE) imaging. In this study, we developed a 3D balanced steady-state free precession (bSSFP) urea-specific imaging sequence: urea frequency-selective RF excitation pulse (Figure 5A), which consists of urea frequency-selective RF excitation pulses interleaved with stack-of-spiral readouts8. This sequence was designed to improve the efficient use of polarization, minimize perturbation to other metabolites, and mitigate off-resonance artifacts (each readout duration = 4 ms), and implemented on commercially available software. Compared to single-shot spiral GRE images of urea, image blurring is considerably reduced in bSSFP images (Figure 5B).
To achieve simultaneous metabolic and perfusion imaging, pyruvate, lactate, and alanine were imaged using multi-slice spiral GRE sequences as previously reported8, interleaved with urea 3D bSSFP sequence. Adult rats (n = 3) were injected with 2.5ml co-polarized pyruvate and urea produced using forementioned SOPs, then imaged with volumetric converge and 4.2s temporal resolution. A panel of representative images and HP signal dynamics are shown in Figure 5C, D.
Summary
We developed a co-polarization system and a standard operating procedure (SOP) that can reproducibly generate sterile HP 13C pyruvate and urea solutions for human injection, as well as a novel imaging approach for simultaneous metabolic and perfusion imaging. Upon FDA approval, this work would lead to the first-in-human dual-agent HP MR study and the first non-pyruvate HP clinical study.Acknowledgements
This work was supported by research grants from the National Institute of Health (NIH): R01CA214554 (JK), P41EB013598(DV, JK). HQ acknowledges graduate fellowship support from UCSF Graduate Division.References
1. Kurhanewicz, J. et al. Hyperpolarized 13C MRI: path to clinical translation in oncology. Neoplasia 21, 1–16 (2019).
2. Wilson, D. M. et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J. Magn. Reson. 205, 141–147 (2010).
3. Lau, A. Z., Miller, J. J., Robson, M. D. & Tyler, D. J. Simultaneous assessment of cardiac metabolism and perfusion using copolarized [1-13 C]pyruvate and 13 C-urea. Magn. Reson. Med. 77, 151–158 (2017).
4. Chen, H.-Y. et al. Assessing Prostate Cancer Aggressiveness with Hyperpolarized Dual-Agent 3D Dynamic Imaging of Metabolism and Perfusion. Cancer Res. 77, 3207–3216 (2017).
5. Bok, R. et al. The Role of Lactate Metabolism in Prostate Cancer Progression and Metastases Revealed by Dual-Agent Hyperpolarized 13C MRSI. Cancers (Basel) 11, (2019).
6. Qin, H. et al. Simultaneous Metabolic and Perfusion Imaging Using Hyperpolarized 13C MRI Can Evaluate Early and Dose-Dependent Response to Radiation Therapy in a Prostate Cancer Mouse Model. Int. J. Radiat. Oncol. Biol. Phys.(2020). doi:10.1016/j.ijrobp.2020.04.022
7. Lee, J. E. et al. Assessing high-intensity focused ultrasound treatment of prostate cancer with hyperpolarized 13 C dual-agent imaging of metabolism and perfusion. NMR Biomed. e3962 (2018). doi:10.1002/nbm.3962
8. Tang, S. et al. A metabolite-specific 3D stack-of-spiral bSSFP sequence for improved lactate imaging in hyperpolarized [1-13 C]pyruvate studies on a 3T clinical scanner. Magn. Reson. Med. (2020). doi:10.1002/mrm.28204
Figures