0219
Low-Field Non-Contrast Cardiopulmonary MRI for Morphologic and Functional Assessment in Post-Covid Patients1Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Medical Solutions, New York, NY, United States, 3Pulmonary Medicine, NYU Langone Health, New York, NY, United States, 4National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Low-field (.55T) non-contrast MRI was performed in 15 post-Covid patients for combined cardiac and pulmonary evaluation, allowing for derivation of cardiac function in terms of left ventricular ejection fraction, as well as assessment for presence of persisting pulmonary parenchymal abnormalities. As the number of post-Covid patients increases, a radiation and contrast-free mode of cardiopulmonary imaging is of increasing relevance; low-field MRI in particular is a promising tool for high-performance lung imaging.
Introduction
As the cohort of post-Covid patients continues to increase, many patients may experience persistent “long-Covid” or “long-hauler” symptoms1, which may differ based on severity of initial presentation or factors such as ICU admission. Post-Covid patients may present for serial radiograph or CT exams to document resolution of groundglass abnormalities, or to assess evolution of organizing, fibrosis-like opacities. The time to resolution for pulmonary parenchymal abnormalities is not widely understood; a study of 103 post-Covid patients demonstrated persisting CT abnormalities in one fourth of patients at 3 months post admission2. Another investigation of 134 CT scans in post-Covid patients found nearly 55% to demonstrate parenchymal abnormalities, most commonly groundglass opacities and fibrosis3.Prior studies have described pulmonary opacities on MRI in Covid-19 patients4, including based on axial UTE sequences5, and a case report on low-field MRI6. Lung parenchyma typically suffers from poor signal intensity due to low proton density and susceptibility artifact from air. Low-field MRI may be uniquely applicable to the imaging of lung parenchyma due to less susceptibility at air tissue interfaces, which may result in greater parenchymal signal.
Objective
To assess the feasibility of low-field strength (0.55T) MRI for cardiopulmonary imaging assessment in post-Covid patients.Methods
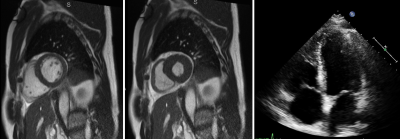
A commercial MRI System (1.5T MAGNETOM Aera; Siemens Healthcare, Erlangen, Germany) was modified to operate as a prototype system at 0.55T field strength7. Non-contrast cardiopulmonary images were acquired in 15 post-Covid patients from 9/8/2020-10/22/2020. Cardiac protocol included short axis and four-chamber retrospectively-gated breath hold (BH) cine imaging, retrospectively-gated BH GRE imaging of the aortic valve, and aortic and pulmonary flow quantification. Pulmonary imaging sequences included coronal and axial BH trufisp, 2D coronal free-breathing trufisp, axial navigator-triggered T2 Blade, and spiral ultra-short echo time (UTE) BH images.Left ventricular ejection fraction (EF) was derived from MR images, and compared to echocardiography-derived EF. The presence or absence of pulmonary parenchymal abnormality was graded by lobe on MRI, and on most proximate preceding companion CT chest. Concordance between MRI and CT chest for the detection of pulmonary parenchymal abnormality was assessed. Presence of clinical symptoms, including dyspnea, cough, wheeze and exhaustion, was assessed by questionnaire near time of MR imaging.
Results
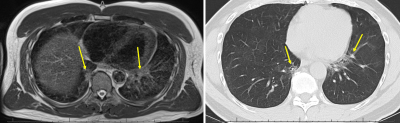
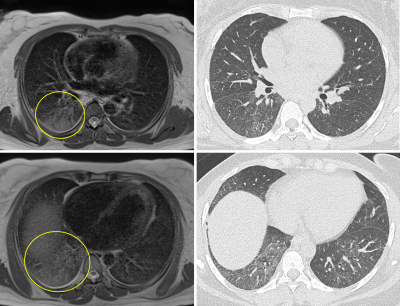
11 men and 4 women were included, mean age 52 (range 19-76 years). Average time between MRI and echocardiography was 68 days (range 20-238 days). Low-field strength MRI allowed quantification of left ventricular ejection fraction, with average LVEF 63%, range 48-70%. LVEF by MRI was normal in 13/15 patients, and mildly reduced in 2 patients. LVEF ranged from 60-70% on echocardiography, within normal range for all patients.MRI was obtained an average of 61 days after CT chest imaging in 13 patients (range 2-145 days), and preceded CT imaging in the remaining 2 patients. A fellowship-trained cardiothoracic radiologist reviewed CT exams for the presence of pulmonary parenchymal abnormalities including consolidation and reticular or groundglass opacities. Parenchymal abnormality was present in 12/13 patients with chest CT preceding MRI, and classified by lobar involvement (7 RUL, 7 RML, 10 RLL, 6 LUL, 10 LLL). Pulmonary parenchymal abnormalities were identified on follow-up MRI in 9/13 patients (5 RUL, 5 RML, 8 RLL, 4 LUL, 8 LLL), with findings persisting on several MRIs at intervals greater than 3 months. There was high correlation between MRI and CT findings (r=.73) despite potential for resolution of some parenchymal findings between initial CT and follow-up MRI.
Clinical symptoms were assessed within 0-36 days of MRI exam acquisition. Eight of 15 patients reported presence of one or more clinical symptom: 7/15 (47%) reporting exhaustion; 3/15 cough; 2/15 dyspnea; and 1/15 wheeze. There was no significant difference between symptomatic and asymptomatic groups in terms of extent of lung involvement on MRI.
Discussion
We found pulmonary parenchymal abnormalities on CT in 93% of our post-Covid patients, and 73% (11/15) of MRIs in our post-Covid cohort. Our findings demonstrate low-field MRI is a non-invasive means of cardiac function quantification, and even without contrast, can identify persisting pulmonary parenchymal abnormalities. Further refinement is necessary to optimize slice thickness to more closely mirror that of clinically acquired CT (T2 blade acquisitions were at 6 mm), and to maximize T2 signal while minimizing artifact to increase radiologist confidence in true lung findings.Continued study is expected to further augment the utility of cardiopulmonary MRI, including for the assessment of perfusion or ventilation defects8–10. For example, reduced pulmonary peripheral vessel blood volume has been shown by CT in Covid-19 patients in comparison to healthy controls and matched ARDS patients11. Ventilation defects have also been demonstrated in post-Covid-19 patients by xenon-enhanced hyperpolarized MRI12. More clinically applicable will be incorporation of noncontrast-enhanced free-breathing assessment of pulmonary perfusion and ventilation defects on phase-resolved functional lung MRI8–10, which may also potentially be done on low-field MRI.
Conclusion
MRI is a useful tool for radiation and contrast-free morphologic and functional cardiopulmonary surveillance in post-Covid patients, who may have persisting clinical symptoms and imaging findings of yet unclear significance and duration.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing research agreement between NYU and Siemens Healthcare.References
1. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J [Internet] 2020 [cited 2020 Dec 14];Available from: http://www.ncbi.nlm.nih.gov/pubmed/33303539
2. Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J [Internet] 2020 [cited 2020 Dec 14];Available from: http://www.ncbi.nlm.nih.gov/pubmed/33303540
3. Zhang S, Liu L, Yang B, et al. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir Res [Internet] 2020 [cited 2020 Dec 14];21(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33243228/
4. Ates OF, Taydas O, Dheir H. Thorax Magnetic Resonance Imaging Findings in Patients with Coronavirus Disease (COVID-19). Acad Radiol [Internet] 2020 [cited 2020 Dec 14];27(10):1373–1378. Available from: https://pubmed.ncbi.nlm.nih.gov/32830031/
5. Yang S, Zhang Y, Shen J, et al. Clinical Potential of UTE-MRI for Assessing COVID-19: Patient- and Lesion-Based Comparative Analysis. J Magn Reson Imaging [Internet] 2020 [cited 2020 Dec 14];52(2):397–406. Available from: /pmc/articles/PMC7300684/?report=abstract
6. Heiss R, Grodzki DM, Horger W, Uder M, Nagel AM, Bickelhaupt S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging [Internet] 2021 [cited 2020 Dec 14];76:49–51. Available from: https://pubmed.ncbi.nlm.nih.gov/33220447/
7. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology [Internet] 2019 [cited 2020 Dec 3];293(2):384–393. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2019190452
8. Klimeš F, Voskrebenzev A, Gutberlet M, et al. Free‐breathing quantification of regional ventilation derived by phase‐resolved functional lung (PREFUL) MRI. NMR Biomed [Internet] 2019 [cited 2020 Dec 14];32(6):e4088. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/nbm.4088
9. Behrendt L, Voskrebenzev A, Klimeš F, et al. Validation of Automated Perfusion-Weighted Phase-Resolved Functional Lung (PREFUL)-MRI in Patients With Pulmonary Diseases. J Magn Reson Imaging [Internet] 2020 [cited 2020 Dec 14];52(1):103–114. Available from: https://pubmed.ncbi.nlm.nih.gov/31872556/
10. Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med [Internet] 2018 [cited 2020 Dec 14];79(4):2306–2314. Available from: https://pubmed.ncbi.nlm.nih.gov/28856715/
11. Thillai M, Patvardhan C, Swietlik EM, et al. Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19. Thorax [Internet] 2020 [cited 2020 Dec 14];Available from: https://thorax.bmj.com/content/early/2020/08/28/thoraxjnl-2020-215395
12. Li H, Zhao X, Wang Y, et al. Damaged lung gas-exchange function of
discharged COVID-19 patients detected by hyperpolarized 129 Xe MRI . Sci Adv
[Internet] 2020 [cited 2020 Dec 14];eabc8180. Available from:
https://pubmed.ncbi.nlm.nih.gov/33219111/
Figures