0217
Examining cerebral blood flow in adults with COVID-19
William S.H. Kim1,2, Xiang Ji2, J. Jean Chen1,3, Asaf Gilboa3, Eugenie Roudaia3, Allison Sekuler3, Aravinthan Jegatheesan4, Mario Masellis2, Benjamin Lam2, Robert Fowler5, Chris Heyn2, Sandra E. Black2, Simon J. Graham1,4, and Bradley J. MacIntosh1,2
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, ON, Canada, 3Rotman Research Institute, Toronto, ON, Canada, 4Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada, 5Evaluative Clinical Sciences, Trauma, Emergency & Critical Care, Sunnybrook Research Institute, Toronto, ON, Canada
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Hurvitz Brain Sciences Program, Sunnybrook Research Institute, Toronto, ON, Canada, 3Rotman Research Institute, Toronto, ON, Canada, 4Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada, 5Evaluative Clinical Sciences, Trauma, Emergency & Critical Care, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
The relationship between coronavirus disease 2019 (COVID-19) and cerebral blood flow (CBF) is not well understood. Here, we report on CBF measured by pseudo-continuous arterial spin labeling among adults that weeks prior had experienced flu-like symptoms with either a positive or negative COVID-19 diagnosis. Recruitment is ongoing, but at present we report no group differences in CBF across brain grey matter. However, subsequent regional analyses point to possible CBF abnormalities in those with a positive COVID-19 diagnosis.
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic continues to unfold, there is growing evidence of multi-organ pathophysiology in some individuals.1 Similar to previous human coronaviruses, COVID-19 has neuroinvasive capabilities and has been linked to a wide range of neurological complications.2–4 However, comparatively less is known regarding the time course for physiological changes that may accompany such neurological symptoms. Thus, there is a need for longitudinal imaging studies to elucidate the relationship between COVID-19 and cerebral blood flow (CBF), as indexed by magnetic resonance imaging (MRI). Several cross-sectional studies have investigated CBF among severe COVID-19 patients with inconsistent findings.5–11 In two arterial spin labeling (ASL) MRI studies, Chougar et al. found regional CBF abnormalities in 22 out of 46 patients, whereas Helms et al. reported reductions in frontotemporal CBF in 11 patients.5,8 In contrast, Klironomos et al. described no such abnormalities among 19 patients using dynamic susceptibility contrast MRI.10 Moreover, the existence of any longitudinal COVID-19 effects on CBF is unexplored. Here, we report on an ongoing prospective study investigating the impact of COVID-19 on brain health among adult survivors. We examined regional CBF in adults that weeks prior had experienced flu-like symptoms with either a positive or negative COVID-19 diagnosis. We hypothesize that there will be regional CBF changes in COVID-19 positive adults in the weeks following illness when compared to controls.Methods
To date, 34 adults have been recruited that previously experienced flu-like symptoms. This study focuses on the ASL pulse sequence from the comprehensive brain and lung NeuroCOVID19 MRI protocol.12 Pseudo-continuous ASL imaging was acquired using a background-suppressed three-dimensional gradient spin-echo readout (TR/TE = 4100/36.76 ms, spatial resolution, 2.5 × 2.5 × 2.5 mm3, field-of-view 240 × 240 × 120 mm3, 1500 ms labelling duration, and 7 control-label pairs) on a Siemens 3T Prisma system. ASL processing included motion correction, spatial regularization, generation of control-tag difference images, kinetic model inversion, voxel-wise calibration, and registration to Montreal Neurological Institute (MNI) space using tools from the FMRIB Software Library.13,14 Calibrated CBF maps were then smoothed with an isotropic Gaussian kernel of full-width-at-half-maximum of 5 mm. CBF maps were visually inspected for vascular transit time artifacts or abnormal regional CBF patterns. Two analyses of CBF were conducted: 1) lobar, and 2) regional. At the lobar level, mean CBF values were first extracted from frontal, occipital, parietal, and temporal grey matter as defined by the MNI atlas. Two linear regression models were defined to investigate group differences in CBF: Model 1 included COVID-19 status as an explanatory variable; Model 2 additionally covaried for age and sex. The threshold for statistical significance was set at p < 0.05 (unadjusted). At the regional level, mean CBF values were extracted from regions of interest including the medial frontal cortex, posterior cingulate cortex, precuneus, orbitofrontal cortex, and thalamus as defined by the Harvard-Oxford atlas. Regional CBF was normalized by mean grey matter CBF and converted to Z-scores. This procedure allows for the identification of individuals with regional CBF abnormalities outside of the range Z = ±1.69, corresponding to a significance level of p < 0.05.Results
Of the 34 adults recruited to date, ASL data were available for interim analysis of 26 adults (mean age of 42 years, 14 females). 18 were COVID-19 positive (89 ± 33 days after receiving positive diagnosis, mostly self-isolating) and 8 were COVID-19 negative controls. One CBF map from a 57-year-old man in the COVID-19 group at baseline and three-months follow-up are shown in Figure 1. For Model 1, COVID-19 diagnosis was not associated with altered CBF in any of the grey matter lobes (frontal, β = -0.621, SE = 4.885, p = 0.899; occipital, β = 2.830, SE = 4.923, p = 0.571; parietal, β = -0.669, SE = 5.162, p = 0.898; temporal, β = 2.016, SE = 4.227, p = 0.638) (Figure 2). Similarly, COVID-19 diagnosis did not explain a significant amount of variance in Model 2 (frontal, β = -1.728, SE = 4.243, p = 0.688; occipital, β = 1.979, SE = 4.688, p = 0.677; parietal, β = -1.715, SE = 4.718, p = 0.720; temporal, β = 1.308, SE = 4.050, p = 0.750). Age was a significant predictor of CBF in the frontal lobe (β = -0.410, SE = 0.144, p = 0.009) in Model 2. Across the chosen regions of interest, 8 COVID-19 participants had at least one abnormal CBF measurement (6 of 8 had reduced CBF). Four controls had at least one abnormal CBF measurement (1 of 4 had reduced CBF) (Figure 3).Discussion
In this cohort of adults that experienced flu-like symptoms with and without COVID-19, CBF was not altered across the wide territories defined by the cortical grey matter lobes in the weeks following infection. Reports thus far suggest that potential tissue alterations due to COVID-19 are likely to be more regional in nature and, furthermore, spatially heterogeneous across individuals. The regional analysis in the current study suggests that CBF may be reduced in relation to COVID-19 among the five regions of interest examined. Work is ongoing to expand the sample size and test for a group effect with more statistical power.Acknowledgements
The authors wish to thank all study participants. Funding for this project was provided by the Dr. Sandra Black Centre for Brain Resilience & Recovery.References
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782-793.
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690.
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA Neurol. 2020;77(8):1018-1027.
- Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology. 2020;95(13):e1868-e1882.
- Chougar L, Shor N, Weiss N, et al. Retrospective Observational Study of Brain Magnetic Resonance Imaging Findings in Patients with Acute SARS-CoV-2 Infection and Neurological Manifestations. Radiology. 2020;297(3):e313-e323.
- Soldatelli MD, Amaral LF do, Veiga VC, Rojas SSO, Omar S, Marussi VHR. Neurovascular and perfusion imaging findings in coronavirus disease 2019: Case report and literature review. Neuroradiol J. 2020;33(5):368-373.
- Rudilosso S, Esteller D, Urra X, Chamorro Á. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J Stroke Cerebrovasc Dis. 2020;29(8):103974.
- Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382(23):2268-2270.
- Bonardel C, Bonnerot M, Ludwig M, et al. Bilateral Posterior Cerebral Artery Territory Infarction in a SARS-Cov-2 Infected Patient: discussion about an unusual case. J Stroke Cerebrovasc Dis. 2020;29(9):105095.
- Klironomos S, Tzortzakakis A, Kits A, et al. Nervous System Involvement in COVID-19: Results from a Retrospective Consecutive Neuroimaging Cohort. Radiology. 2020;297(3):e324-334.
- Lang M, Buch K, Li MD, et al. Leukoencephalopathy associated with severe COVID-19 infection: Sequela of hypoxemia? Am J Neuroradiol. 2020;41(9):1641-1645.
- Graham SJ, Chen JJ, Gilboa A, et al. Neurocovid19: impact of the virus on the brain. Abstract accepted at: SFN Global Connectome; January, 2021.
- Groves AR, Chappell MA, Woolrich MW. Combined spatial and non-spatial prior for inference on MRI time-series. Neuroimage. 2009;45(3):795-809.
- Alsop DC, Detre JA, Golay X, et al. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magn Reson Med. 2015;73(1):102-116
Figures
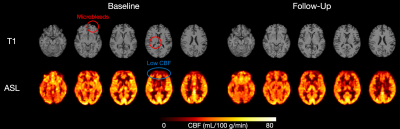
Figure 1. T1-weighted (top) and CBF (bottom) images from a 57-year-old man in the COVID-19 group at baseline (left) and at three-months follow-up (right).
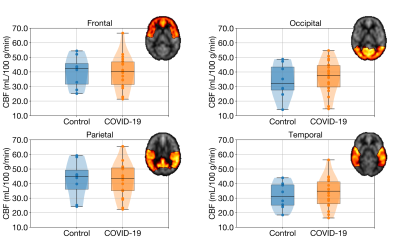
Figure 2. Boxplots of regional CBF in the four grey matter lobes. In both Model 1 and 2, COVID-19 diagnosis did not explain a significant amount of variance in any of the grey matter lobes at an unadjusted significance level of p < 0.05. Age was a significant predictor of CBF in the frontal lobe in Model 2.
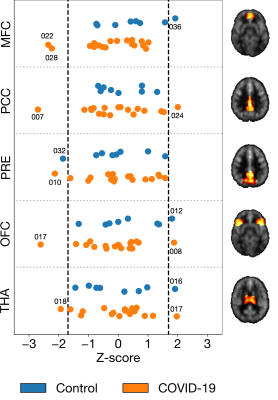
Figure 3. Intensity-normalized regional CBF converted to Z-scores in five selected regions of interest. Z-scores outside of the black dashed lines (indicating Z = ±1.69) were considered to significantly deviate from the mean. Abbreviations: MFC, medial frontal cortex; PCC, posterior cingulate cortex; PRE, precuneus; OFC, orbitofrontal cortex; THA, thalamus.