0211
Infant cerebrospinal fluid dynamics assessed by low b-value diffusion tensor imaging and association with visible Virchow–Robin spaces1Department of Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China
Synopsis
Cerebrospinal fluid (CSF) and interstitial fluid exchange via Virchow Robin space (VRS). It is reasonable to infer that VRS may be related to CSF dynamics. To investigate the association between CSF dynamics and VRS on infants, this work assessed CSF dynamics on infants by DTI with b values of 200 and 1000 s/mm2 and segmented VRS on T2WI images. Results suggest that the ratio between diffusivities derived from low and high b-value DTI could provide complementary information for assessing infant cerebrospinal fluid dynamics. There may exist potential association between Virchow–Robin space volume and cerebrospinal fluid dynamics.
Introduction
Cerebrospinal fluid (CSF) and interstitial fluid (ISF) exchange via the Virchow Robin space (VRS), which forms the key node of the glymphatic system circulation [1]. Therefore, CSF plays important role in mediating the waste clearance pathway in the brain [2]. Visible VRS is common on infants, especially on infants with febrile seizures (FS). Specifically, the inflammatory process (i.e. secretion of cytokine, etc.) on FS infants has been thought to influence the CSF-ISF exchange [3, 4]. It is reasonable to infer that VRS may be related to CSF dynamics. However, the association between CSF dynamics and VRS on infants has not been fully investigated. Low b-value diffusion tensor imaging (DTI) could provide valuable information for assessing CSF dynamics [1, 5]. Therefore, this work investigated CSF dynamics on infants by DTI with b values of 200 and 1000 s/mm2. Furthermore, correlation between the VRS volume and the ratio for mean diffusivity (MD) of low b value (200 s/mm2: MD200) and that of high b value (1000 s/mm2: MD1000) was performed.Materials and methods
This study is approved by the local institutional review board. Informed written consents were obtained from parents of infants.This study enrolled infants with simple FS (SFS) diagnosed according to the criteria defined by American Academic of Pediatric [6, 7]. Infants without any epilepsy or other types of seizures and without abnormalities on magnetic resonance imaging (MRI) were enrolled as controls. The Visible VRS was determined by the visual assessment based on T2 weighted imaging (T2WI) images.
MRI was performed on a 3T scanner (Signa HDxt; GE Healthcare; Milwaukee, Wisconsin, USA) with an 8-channel head coil. The body temperature, the heart rate, the respiration rate, and the transcutaneous oxygen saturation were monitored throughout the MRI procedure. Micro earplugs were placed bilaterally in the external auditory meatus to protect the hearing. T2WI was performed by using a fast spin echo sequence with following parameters: repetition time/echo time = 4200/120 ms; slice thickness = 4 mm; field of view = 240 × 240 mm2; and acquisition matrix = 320 × 320. DTI was performed by using a single shot echo planar imaging sequence with following parameters: b values = 0, 200, 1000 s/mm2; 18 gradient directions per nonzero b value; NEX = 1; repetition time/echo time = 11000/91.7 ms; slice thickness = 4 mm; field of view = 240 × 240 mm2; acquisition matrix = 172 × 172.
FMRIB software library (www.fmrib.ox.au.uk/fsl) was used for processing DTI. MD of different b values (200 and 1000 s/mm2: MD200 and MD1000) were calculated by using the FMRIB Diffusion Toolbox. The ratio between MD200 and MD1000 was calculated by: Ratio = MD200 / MD1000. A custom tool in Matlab (R2012b; MathWorks, Natick, MA, USA) was used to segment VRS in white matter regions above bilateral ventricles (including the bilateral ventricular level) [8, 9]. Inter-group comparisons in age and VRS volume were performed by using Mann-Whitney U test. Inter-group comparisons in gender ratio were performed by using Chi-square test. Pearson correlation was used to analyze the correlation between the VRS volume and the ratio of MD200/MD1000. Tests were considered statistically significant at P < 0.05.
Results
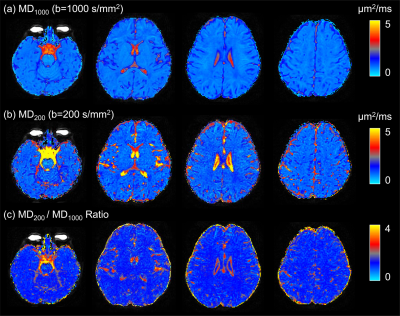
This study enrolled 37 infants (age range: 1.04 ~ 2.91 years), including 12 controls, 10 infants with visible VRS, and 15 infants with visible VRS and SFS. There is no significant difference in age at MRI or gender ratio across groups. SFS infants held larger VRS volume than controls (P<0.01) (Table 1 and Figure 1). Maps of MD200, MD1000, and MD200/MD1000 Ratio held different image contrasts (Figure 2). MD1000 map demonstrated that diffusivity was higher in ventricles. Compared with MD1000, higher values of MD200 indicated that CSF flow contributed to MD200, besides the diffusion effect. Furthermore, higher MD200 could be found in regions nearby the choroid plexus, instead of the whole ventricle. This could be revealed more obviously by the map of MD200/MD1000 Ratio. The MD200/MD1000 Ratio was larger than 1 in the main part of the CSF region (Figure 3). Infants with visible VRS held smaller MD200/MD1000 Ratio than controls in the ratio range of 1.3~2.3 (Figure 3b). Moreover, significant negative correlation could be found between VRS volume and the MD200/MD1000 Ratio (average in the range of 1.3~2.3) (P=0.01).Discussion
Similar to previous findings on CSF dynamics [5], CSF motion is more obvious in the ventral posterior fossa, suprasellar cistern, ambient cistern and Sylvian vallecular, instead of the whole ventricles (Figure 2). The CSF dynamics is the basis of the CSF-ISF circulation [1, 10]. Previous study has suggested that seizures were associated with dilated VRS [11]. This work finds that VRS volumes correlate with CSF dynamics. This may be associated with seizures and cerebral glymphatic circulation [12-15]. Disruption of CSF-ISF exchange could influence the clearance of waste and secretion products, which may aggravate VRS dilation and affect the CSF dynamics [16].Conclusion
The MD200/MD1000 Ratio derived from low and high b-value DTI could provide complementary information for assessing infant CSF dynamics. Results here provides clues for investigating association between VRS volume and CSF dynamics.Acknowledgements
Acknowledgments:This study was supported by the National Natural Science Foundation of China (81901823, 81971581, and 81901516), the Innovation Team Project of Natural Science Fund of Shaanxi Province (2019TD-018), National Key Research and Development Program of China (2016YFC0100300). Please address correspondence to Xianjun Li, e-mail: xianj.li@mail.xjtu.edu.cn, and Jian Yang, e-mail: yj1118@mail.xjtu.edu.cn.References
1. Plog, B.A. and M. Nedergaard, The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol, 2018. 13: p. 379-394.
2. Harrison, I.F., et al., Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife, 2018. 7: p. e34028.
3. Marchi, N., M. Banjara, and D. Janigro, Blood–brain barrier, bulk flow, and interstitial clearance in epilepsy. Journal of Neuroscience Methods, 2016. 260: p. 118-124.
4. Chung, S., Febrile seizures. Korean J Pediatr, 2014. 57(9): p. 384-95.
5. Toshiaki, T., et al., Multi b-value diffusion weighted image diphase map (MbDDM) to evaluate cerebrospinal fluid dynamics. Proceedings of International Society of Magnetic Resonance in Medicine (ISMRM) 28th Annual Meeting 2020: p. 0537.
6. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures American Academy of Pediatrics, Febrile Seizures: Clinical Practice Guideline for the Long-term Management of the Child With Simple Febrile Seizures. Pediatrics, 2008. 121(6): p. 1281-1286.
7. Laino, D., E. Mencaroni, and S. Esposito, Management of Pediatric Febrile Seizures. Int J Environ Res Public Health, 2018. 15(10): p. 2232.
8. Cai, K., et al., The feasibility of quantitative MRI of perivascular spaces at 7T. J Neurosci Methods, 2015. 256: p. 151-156.
9. Ballerini, L., et al., Perivascular Spaces Segmentation in Brain MRI Using Optimal 3D Filtering. Sci Rep, 2018. 8(1): p. 2132.
10. Nedergaard, M., Neuroscience. Garbage truck of the brain. Science, 2013. 340(6140): p. 1529-1530.
11. Liu, C., et al., Quantification of visible Virchow–Robin spaces for detecting the functional status of the glymphatic system in children with newly diagnosed idiopathic generalized epilepsy. Seizure, 2020. 78: p. 12-17.
12. Dube, C.M., A.L. Brewster, and T.Z. Baram, Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev, 2009. 31(5): p. 366-371.
13. Gorter, J.A., E.A. van Vliet, and E. Aronica, Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav, 2015. 49: p. 13-16.
14. Ha, J., et al., Interleukin-4 and tumor necrosis factor-alpha levels in children with febrile seizures. Seizure, 2018. 58: p. 156-162.
15. Koh, S., Role of Neuroinflammation in Evolution of Childhood Epilepsy. J Child Neurol, 2018. 33(1): p. 64-72.
16. Dadas, A., J. Washington, and D. Janigro, Cerebral Waste Accumulation and Glymphatic Clearance as Mechanisms of Human Neurological Diseases. J Neurol Neuromedicine, 2016. 1(7): p. 15-19.
Figures