0205
Characterization of Apparent Exchange Rate in Human Brain White Matter1Interdisciplinary Institute of Neuroscience and Technology (ZIINT), School of Medicine, Zhejiang University, Hangzhou, China, HangZhou, China, 2College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China, HangZhou, China, 3MR Collaboration, Siemens Healthcare, Shanghai, China, ShangHai, China
Synopsis
Filter exchange imaging (FEXI) is a non-invasive method to measure water exchange among diffusion compartments by apparent exchange rate (AXR). However, in human white matter, it is still controversial whether the diffusion-encoding gradient direction will affect AXR measurement. In this study, we performed FEXI on human brain with 20 diffusion gradient directions to explore features of AXR in white matter. We found that AXR measured with diffusion direction perpendicular to fiber (~ 0.47 s-1) is significantly larger than that parallel to fiber (~ 0.15 s-1), suggesting FEXI at different directions might measure exchanges between different biological compartments in white matter.
Introduction
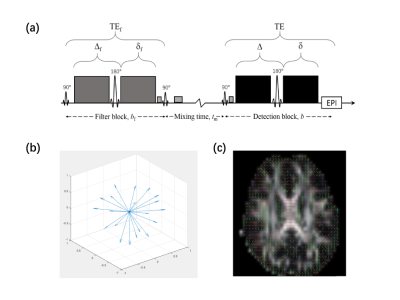
Filter-exchange imaging (FEXI), a special case of diffusion exchange spectroscopy adapted for clinical applications, has the potential to reveal different physiological water exchange processes1, 2. A recent study has found that modulating the amplitude of the diffusion filter (bf) strength and detection blocks in FEXI could reveal distinct apparent exchange rate (AXR) contrast in brain tissues3. However, it is still controversial whether modulating diffusion encoding gradient direction can affect the AXR measurements, especially in white matter where water diffusion shows strong anisotropy. A recent study on ex-vivo monkey brain tissue found that AXR in white matter shows strong diffusion-encoding direction dependence4. However, the direction dependence of AXR was found to be small and non-significant in human white matter in-vivo, though only three direction-encoding directions were explored1. In this study, we further explore the diffusion-encoding direction dependence of FEXI in human brain white matter by performing FEXI with 20 diffusion-encoding directions in-vivo.Methods
Six healthy subjects (age 22±2 years) were recruited and received MRI scans with a 3.0 T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). MRI scans included 3D SPACE T2-weighted images (1.0×1.0×1.0 mm3 resolution), DTI and FEXI. The DTI was acquired with 4.0×4.0×4.0 mm3 resolution, 20 slices, b = 0s/mm2 (2 repetitions), b = 1000s/mm2 (20 directions). FEXI (illustrated in Figure 1a) was performed with bf = 830s/mm2 and two b values in detection block (bd = 100s/mm2 with 3 repetitions and 1300s/mm2 with 6 repetitions), resolution 4.0×4.0×4.0 mm3, 12 slices. The directions of bf and bd were always kept the same and acquired along 20 diffusion-encoding directions (Figure 1b). And three mixing times (tm): 25, 200, 400ms were set. FEXI was also acquired with bf = 0s/mm2 and shortest (25ms), as the equilibrium data for fitting.All DTI and FEXI data underwent pre-processing including motion and eddy current distortion correction in TORTOISE5. After pre-processing, the DTI data were fit to the non-linear DTI model in TORTOISE to calculate FA, MD, and primary eigenvector. For FEXI data, ADC'(tm) were computed for each diffusion gradient direction, according to $$ADC'(t_{m}) = -\frac{1}{b_{2}-b_{1}}\ln(\frac{s(t_{m},b_{2})}{s(t_{m},b_{1})}) [1]$$ where b1 and b2 were the two bd values, s(tm, b1) and s(tm, b2) are FEXI signals at b1 and b2, respectively. Then, the calculated ADC'(tm) at the three tm and bf = 0 were fitted to $$ADC'(t_{m}) = ADC(1-\sigma\exp(-t_{m}AXR)) [2]$$ to obtain AXR, filter efficiency (σ), equilibrium ADC.
Voxels with FA and MD values in the range of 0.35-1.0 and 10-1000 μm2/s, respectively, was identified as white matter which were further divided into three groups based on FA values (WM1, 0.35<FA<0.5; WM2, 0.5<FA<0.65; WM3, 0.65<FA<1). In addition, the subcortical striatum was also selected to compare with WM. For each voxel, the angle (ϑ) between diffusion-encoding directions of FEXI and the primary eigenvector were computed. FEXI with less than 15° and 75°-90° were considered as parallel and perpendicular to the primary eigenvector (i.e., axon direction), respectively, and averaged to compute AXR, σ and ADC.
Results and Discussion
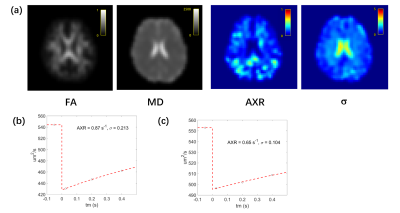
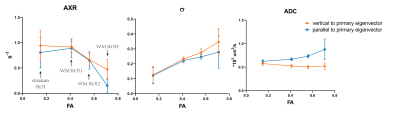
Before exploring the anisotropy of AXR, we averaged the FEXI data of 20 diffusion-encoding gradients and fitted the data with Eq. [1] and [2]. The results of one subject are shown in Figure 2. Excellent fitting can be observed on both white matter and subcortical striatum ROI (Figure 2(b, c)). The AXR in white matter of all subjects is 0.79 s-1, which is very close to the previous study (mean AXR in white matter ROIs is 0.85 s-1)6. The in white matter in this study is 0.23, which is in the same range of that reported previously3.To explore the anisotropy in FEXI, we firstly studied FEXI in WM3 (FA > 0.65) where the potential effect of crossing fiber is relatively small. Significant difference of AXR, σ and ADC was detected between the diffusion gradient direction perpendicular to axon and that parallel to axon (Figure 3). The ADC results are not surprising as the well-known anisotropic apparent diffusivity in white matter. The results ofAXR show agreement with the previous study on ex-vivo WM sample4. Previous studies have also found that the biexponential representation of single PGSE data in human WM also shows direction dependence7, 8. When taking those values into the calculation of σ (detailed equations in reference9 ), they could well explain the direction-dependence of σ found here. In white matter, there are more than two water compartments (intra-axonal space, extracellular space, astrocytes, etc.). When the diffusion filter placed along fibers, it is mostly likely that the intra-axonal magnetization gets filtered due to their higher diffusivity and AXR measures water exchange between intra- and extra-axonal water. In this case, the myelin structure could potentially make this exchange process very slow. On the other hand, when the diffusion filter placed perpendicular to fibers, it could potentially filter out the extracellular or intra-astrocytes water showing relatively faster diffusivity and AXR might measure the transmembrane water exchange associated with astrocytes. In addition, we found the anisotropy of AXR, σ, and ADC becomes smaller as FA decreases (Figure 4).
Conclusion
In this study, we demonstrate the anisotropy of AXR in human white matter. The AXR measured along different directions might reflect different water exchange processes between different microstructural compartments.Acknowledgements
References
1. Nilsson M, Latt J, van Westen D, et al. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging. Magn Reson Med 2013; 69: 1573-1581. 2012/07/28. DOI: 10.1002/mrm.24395.
2. Lasič S, Oredsson S, Partridge SC, et al. Apparent exchange rate for breast cancer characterization. 2016; 29: 631-639. DOI: https://doi.org/10.1002/nbm.3504.
3. Bai R, Li Z, Sun C, et al. Feasibility of filter-exchange imaging (FEXI) in measuring different exchange processes in human brain. Neuroimage 2020; 219: 117039. 2020/06/14. DOI: 10.1016/j.neuroimage.2020.117039.
4. Sonderby CK, Lundell HM, Sogaard LV, et al. Apparent exchange rate imaging in anisotropic systems. Magn Reson Med 2014; 72: 756-762. 2013/10/15. DOI: 10.1002/mrm.24957.
5. Pierpaoli C, Walker L, Irfanoglu M, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. ISMRM 18th Annual Meeting 2010.
6. Lampinen B, Szczepankiewicz F, van Westen D, et al. Optimal experimental design for filter exchange imaging: Apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magn Reson Med 2017; 77: 1104-1114. 2016/03/13. DOI: 10.1002/mrm.26195.
7. Clark CA and Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magnetic Resonance in Medicine 2000; 44: 852-859. DOI: 10.1002/1522-2594(200012)44:6<852::Aid-mrm5>3.0.Co;2-a.
8. Maier RVMRLRSJHDMSE. Diffusion Sensitization Direction Dependence of Biexponential Diffusion Decay Parameters in the splenium. ISMRM conference 2009.
9. Lasič S, Nilsson M, Lätt J, et al. Apparent exchange rate mapping with diffusion MRI. Magnetic Resonance in Medicine 2011; 66: 356-365. DOI: 10.1002/mrm.22782.
Figures