0203
Age-dependent effects of methylphenidate on emotional dysregulation: an RCT in stimulant treatment-naïve male ADHD patients1Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Child Study Center, Accare, Groningen, Netherlands, 3Dutch Autism and ADHD research center, University of Amsterdam, Amsterdam, Netherlands, 4Department of Child and Adolescent Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 5De Bascule, Academic Centre for Child and Adolescent Psychiatry, Amsterdam, Netherlands, 6Brain Plasticity Group, Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, Amsterdam, Netherlands, 7Division of Psychosocial Research and Epidemiology, Netherlands Cancer Institute, Amsterdam, Netherlands
Synopsis
Emotional dysregulation (ED) is an important outcome moderator of Attention-Deficit/Hyperactivity Disorder (ADHD). We previously found that acute administration of methylphenidate age-dependently modulated neural mechanisms underlying ED, i.e., amygdala reactivity, but effects of chronic methylphenidate administration remain unknown. Following randomization to 16 weeks of methylphenidate or placebo treatment, we here report a lasting improvement in ED, depressive and anxiety symptoms in ADHD children, whereas a transient improvement of ED and depressive symptoms occurred in adults, independent of treatment condition. Although depressive and anxiety symptoms at baseline negatively predicted ADHD symptom change in adults, age-dependent effects on amygdala reactivity were absent.
Introduction
Methylphenidate, the primary pharmacological treatment for Attention-Deficit/Hyperactivity Disorder (ADHD), effectively alleviates symptoms of inattention and hyperactivity in patients with ADHD. However, it has become clear that patients with ADHD also present emotional regulation problems, independent of other comorbidities4,5. Evidence has emerged that emotional dysregulation (ED) negatively predicts the methylphenidate treatment response, with higher levels of ED, generally predicting lower treatment efficacy6. Nevertheless, although methylphenidate may positively affect ED, empirical support remains anecdotal7. Increasing (pre-)clinical evidence suggests that the effects of methylphenidate may depend on the age of first exposure1. For example, methylphenidate taken during adolescence can, e.g., induce anxiety and depressive-like behavior8, and increase impulsivity during adulthood9. Furthermore, we previously showed age-dependent effects of methylphenidate on the dopamine system in ADHD patients2 and found acute methylphenidate administration to age-dependently modulate one of the functional neural mechanisms underlying ED, i.e., amygdala reactivity3. However, the effects of chronic methylphenidate administration on such measures remain unknown. As it remains debated which neural mechanisms underlie the improvements in ED in ADHD after stimulant treatment, we set out to investigate the chronic methylphenidate treatment effects on clinical measures and functional neural mechanisms of ED, and if these effects are modulated by age.Methods
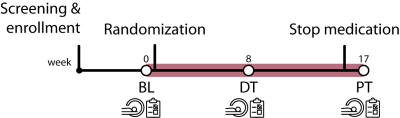
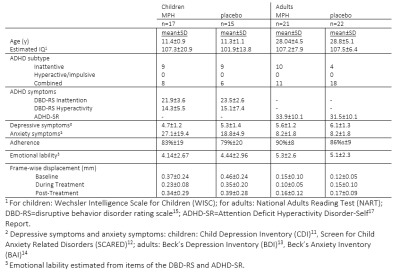
Data was used from the ‘effects of Psychotropic drugs On Developing brain-methylphenidate’ (‘ePOD-MPH’) randomized clinical trial, a 16-week double-blind, randomized, placebo-controlled, multicenter trial with methylphenidate in stimulant treatment-naive patients with ADHD10 (NL34509.000.10; NTR3103). We included 50 boys (10-12 years of age) and 49 men (23-40 years of age) in the ePOD-MPH trial. All patients and parents or legal representatives of the children provided written informed consent. Symptoms of ADHD, depression, anxiety, and emotion lability were assessed using self-reported questionnaires (children: DBD-RS15, CDI11, SCARED12; adults: ADHD-SR17, BDI13, BAI14). ED specifically was evaluated by extracting specific items from the ADHD questionnaires in accordance with Sobanski et al.16. To assess amygdala reactivity, we measured fMRI activity during an emotion face-matching task18 at three times points: baseline (BL), eight weeks during treatment (DT), and one week after the trial end (post-treatment (PT))(Figure 1). This block design consisted of emotional blocks with angry and fearful face stimuli and neutral blocks with ellipses assembled from scrambled faces. The MRI study was performed on a 3T Philips scanner (Philips Healthcare, Best, The Netherlands) using an 8-channel receive-only head coil. FMRI data were acquired using a single-shot echo-planar imaging sequence (parameters: TR/TE=2300/30ms, resolution=2.3×2.3×3mm, 39 sequential slices, FA=80°, dynamics=70). Preprocessing was performed using FMRIPREP v1.2.319,20. fMRI data were entered into the first-level analysis (FSL/FEAT)21. For our region-of-interest analyses of the face-matching task, mean signal intensity for the left and right amygdala were extracted22. To explore whole-brain activity in the main task contrasts (faces vs. shapes; shapes vs. faces), the first-level contrast-of-parameter-estimates (COPE) maps were analyzed using non-parametric permutation testing (5000 permutations) in FSL Randomise. Linear mixed-effects models were used to investigate the effects of time (BL, DT, PT), medication (placebo, methylphenidate), and age group (children, adults) on fMRI activity and clinical variables. Exploratory prediction analyses were done using linear models.Results
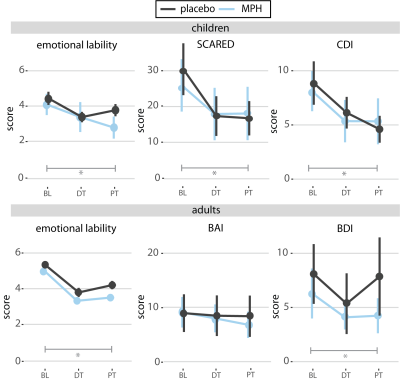
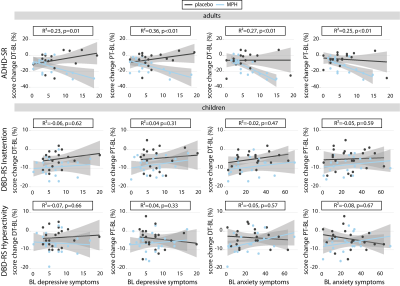
Treatment groups did not differ in age, IQ, depressive or anxiety symptoms, nor in ADHD severity at baseline (Table 1). We did not show a significant age x medication x time interaction for ADHD, anxiety, depressive, or ED symptoms. ADHD symptoms showed an interaction effect of medication and time, indicating more improvement in the methylphenidate compared to placebo groups. Furthermore, we found a main effect of time for depressive (p<0.01) and anxiety symptoms (p<0.01) and for emotion lability (p=0.03) in children, and depression (p=0.02) and emotion lability (p<0.01) in adults (Figure 2). No main or interaction effects on left or right amygdala reactivity were found (p>0.05). However, post-hoc tests for the right amygdala reactivity in children showed a significantly higher reactivity in the methylphenidate than placebo condition at DT (p=0.04), which was not observed at PT (Figure 3A). Exploratory whole-brain analyses revealed that methylphenidate increased reactivity to the face-matching task in children from DT to PT in the Superior-Frontal-Gyrus and Paracingulate-Cortex. Additionally, a decrease in reactivity in placebo-treated adults from BL to PT in the Lateral-Occipital-Cortex was shown (Figure 3B). Additionally, prediction analysis showed an age-dependent effect of BL symptomatology on ADHD symptom change, with BL depression and anxiety symptoms being negatively associated with ADHD symptom change in adults, but not in children (DT-BL: depression: p<0.01; anxiety: p<0.01; PT-BL: depression: p<0.01; anxiety: p<0.01) (Figure 4).Discussion
Methylphenidate effectively reduced ADHD symptoms compared to placebo. In contrast, independent of the randomization group, ED, depressive, and anxiety symptoms in ADHD children improved one-week after wash-out (i.e. PT), whereas only a transient (i.e. DT) improvement was found of ED and depressive symptoms in adults. However, we did not find age-dependent effects of chronic methylphenidate on the functional neural mechanisms of ED, as we previously demonstrated for acute methylphenidate administration3. Furthermore, this data suggests that baseline clinical data may be used to predict treatment effects on ADHD symptoms in adults.Acknowledgements
This study was funded by a personal research grant awarded to LR by the Academic Medical Center, University of Amsterdam, and 11.32050.26 ERA-NET PRIOMEDCHILD FP 6 (EU) and a grant from Amsterdam Brain and Cognition (ABC). Suffugium, a Dutch non-profit organization, financially supported MB. AS and PJL are supported by the Urban Mental Health program of the University of Amsterdam. We would like to thank all patients and their parents for participating in this study and all students that helped collecting and analyzing the data.References
1. Andersen SL. Stimulants and the developing brain. Trends Pharmacol. Sci. Elsevier Ltd; 2005. p. 237–243.
2. Schrantee A, Tamminga HGHH, Bouziane C, Bottelier MA, Bron EE, Mutsaerts H-JJMMM, Zwinderman AH, Groote IR, Rombouts SARBA, Lindauer RJJL, Klein S, Niessen WJ, Opmeer BC, Boer F, Lucassen PJ, Andersen SL, Geurts HM, Reneman L. Age-Dependent Effects of Methylphenidate on the Human Dopaminergic System in Young vs Adult Patients With Attention-Deficit/Hyperactivity Disorder. JAMA Psychiatry American Medical Association; 2016;73:955–962.
3. Bottelier MA, Schrantee A, Ferguson B, Tamminga HGH, Bouziane C, Kooij JJS, Ruiter MB de, Reneman L. Age-dependent effects of acute methylphenidate on amygdala reactivity in stimulant treatment-naive patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res - Neuroimaging Elsevier Ireland Ltd; 2017;269:36–42.
4. Lenzi F, Cortese S, Harris J, Masi G. Pharmacotherapy of emotional dysregulation in adults with ADHD: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. Jan, 2018 p. 359–367.
5. Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry American Psychiatric AssociationArlington, VA; 2014;171:276–293.
6. Masi G, Fantozzi P, Muratori P, Bertolucci G, Tacchi A, Villafranca A, Pfanner C, Cortese S. Emotional dysregulation and callous unemotional traits as possible predictors of short-term response to methylphenidate monotherapy in drug-naïve youth with ADHD. Compr Psychiatry The Author(s); 2020;100:152178.
7. Posner J, Kass E, Hulvershorn L. Using Stimulants to Treat ADHD-Related Emotional Lability. Curr Psychiatry Rep 2014;16.
8. Bolaños C a., Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry Elsevier Inc.; 2003;54:1317–1329.
9. Somkuwar SS, Kantak KM, Bardo MT, Dwoskin LP. Adolescent methylphenidate treatment differentially alters adult impulsivity and hyperactivity in the Spontaneously Hypertensive Rat model of ADHD. Elsevier Inc.; 2016;141:66–77.
10. Bottelier MA, Schouw MLJ, Klomp A, Tamminga HGH, Schrantee AGM, Bouziane C, et al. The effects of Psychotropic drugs On Developing brain ( ePOD ) study : methods and design. BMC Psychiatry 2014;14:48.
11. Kovacs M. The Children’s Depression Inventory (CDI). Psychopharmacol Bull 1985;21:995–998.
12. Muris P, Bodden D, Hale W BB& MB. SCARED-NL. Vragenlijst over angst en bang-zijn bij kinderen en adolescenten. Handleiding bij de gereviseerde Nederlandse versie van de Screen for Child Anxiety Related Emotional Disorders. amsterdam: boom test uitgever; 2007.
13. Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. Beck depression inventory (BDI). Arch Gen Psychiatry 1961;4:562–571.
14. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–897.
15. Pelham Jr. WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders.[erratum appears in J Am Acad Child Adolesc Psychiatry 1992 Nov;31(6):1177]. J Am Acad Child Adolesc Psychiatry 1992;31:210–218.
16. Sobanski E, Banaschewski T, Asherson P, Buitelaar J, Chen W, Franke B, Holtmann M, Krumm B, Sergeant J, Sonuga-Barke E, Stringaris A, Taylor E, Anney R, Ebstein RP, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone S V. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): Clinical correlates and familial prevalence. J Child Psychol Psychiatry Allied Discip 2010;51:915–923.
17. Kooij J. Adult ADHD: Diagnostic assessment and treatment. London: Springer-Verlag; 2012.
18. Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002;17:317–323.
19. Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods Nature Publishing Group; 2019;16:111–116.
20. Esteban O, Ciric R, Finc K, Blair RW, Markiewicz CJ, Moodie CA, Kent JD, Goncalves M, DuPre E, Gomez DEP, Ye Z, Salo T, Valabregue R, Amlien IK, Liem F, Jacoby N, Stojić H, Cieslak M, Urchs S, Halchenko YO, Ghosh SS, La Vega A De, Yarkoni T, Wright J, Thompson WH, Poldrack RA, Gorgolewski KJ. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat Protoc Nature Publishing Group; 2020;1–17.
21. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790.
22. Posner J, Nagel BJ, Maia T V., Mechling A, Oh M, Wang Z, Peterson BS. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011;50:1–19.
Figures