0202
Disparate Cognitive Patterns Captured by Subcortical Profiles in Schizophrenia1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Division of Psychiatry Research, Zucker Hillside Hospital, Glen Oaks, NY, United States, 3Center for Psychiatric Neuroscience, Feinstein Institute for Medical Research, Manhasset, NY, United States
Synopsis
Subcortical morphological abnormalities are associated with cognitive impairment in schizophrenia. We hypothesized that patients with different degree of cognitive impairment might be separated by subcortical morphological abnormalities. Here we identified two distinct clusters in patients with schizophrenia based on their regional subcortical volume. Different degree of regional subcortical volume, global brain volume and cognitive impairment were observed between two clusters of patients, with more severe cognitive impairment in the more severe morphological deficit cluster. These findings indicate critical relationships between subcortical structures and cognition in schizophrenia, and suggest that subcortical morphological abnormalities could help to capture cognitive profiles in schizophrenia.
Introduction
Subcortical morphological alterations in schizophrenia are heterogeneous, reflected by both inconsistent findings1, 2 and great variability3, 4 of regional subcortical volume. Brain morphology-based subtyping studies observed distinct subtypes based on morphological features from whole brain5 or cortical regions6, 7 in schizophrenia. However, little was known that whether different morphological patterns would be existed when only regional subcortical features are employed for stratification given the heterogeneity of subcortical structures. Additionally, subcortical morphological alterations are associated with cognitive impairment in schizophrenia8, 9, which is considered as a core feature of this disorder10. Similar to subcortical alterations, cognitive impairment in patients with schizophrenia are not homogeneous. Distinct cognitive subtypes were reported in cognition-based subtyping studies11-15, some of which showed different degree of subcortical morphological alterations across cognitive clusters14, 15. However, it remains elucidate that whether patients with different degree of cognitive impairment could be separated by subcortical morphological profiles. Therefore, in this study, we aimed to stratify patients with schizophrenia based on regional subcortical volume and to compare their cognitive function after stratification.Methods
Subjects: We included 96 antipsychotic-treated patients with chronic patients in clustering analysis. We also included 35 never-treated patients with chronic schizophrenia and 131 healthy controls to compare with these treated patients in structural and cognitive profiles. The diagnosis of schizophrenia was based on the Structured Clinical Interview of DSM-IV. Chronic schizophrenia was defined as illness duration greater than or equal to 60 months. To relatively reduce the influences of medication confounders, we restricted treated patients to those who only received atypical antipsychotic treatment. The non-patient edition of SCID was used to identify healthy comparison subjects.Cognitive function assessment: The Brief Assessment of Cognition in Schizophrenia (BACS)16, composed of subset scores in six cognitive domains including verbal memory, working memory, motor speed, verbal fluency, attention and speed of information processing and executive functioning and a composite score, was used to evaluate cognitive function in treated patients with chronic patients and healthy controls. BACS scores were not available in never-treated patients due to their earlier enrollment.
Structural imaging acquisition and processing: High resolution 3D T1-weighted images were acquired from all participants on a 3.0 T General Electric EXCITE MR scanner with a spoiled gradient recall sequence and processed by FreeSurfer software. Regional subcortical volume and global brain volume were extracted for clustering analysis and (or) statistical inferences.
Clustering analysis: Subcortical volume of 14 structures (bilateral thalamus, caudate nucleus, putamen, globus pallidus, hippocampus, amygdala and nucleus accumbens) were all employed for clustering analysis in treated patients without any predefined feature selection steps. Regional subcortical volume was regressed out age, sex, illness duration and ICV and subsequently standardized into z scores for k-means clustering analysis with parameter tuning. In order to identify the optimal number of clusters, a summary of 30 indices for clustering assessment were employed, and the final number of clusters was decided by the rule of majority rote.
Statistical analysis: Univariate analysis of covariance (ANCOVA) was performed to compare morphological profiles across identified clusters of treated patients, a group of never-treated patients, and healthy controls with age, sex, intracranial volume (ICV) as covariates. ANCOVA was also employed to assess between-group differences of cognitive function in identified clusters of treated patients and healthy controls with age, sex, educational level as covariates. In order to assess the effect of subcortical volume to cognition in general, ANCOVA was repeated performed on cognitive function with subcortical gray matter volume as an additional covariate.
Results
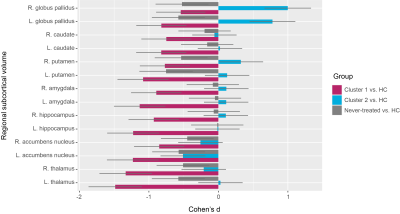
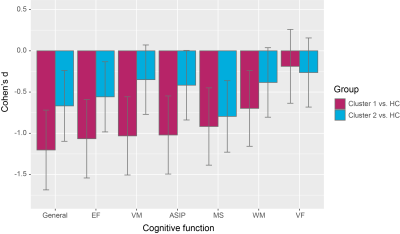
We identified two distinct clusters in patients with schizophrenia based on their regional volume of subcortical structures: one severe deficit cluster displayed extensive deficits with all subcortical regions affected; the other, the moderate abnormal cluster showed enlargement of bilateral globus pallidus and smaller nucleus accumbens (Figure 1).These two clusters of treated patients were not significantly different from each other in demographics or symptoms. Furthermore, these two clusters of treated patients displayed different patterns of cognitive impairment, with more severe impairment of general function, verbal memory, attention and speed of information processing, and executive functioning in the severe deficit cluster (Figure 2). Such significant cognitive differences between two clusters of treated patients disappeared after including subcortical gray matter volume as an additional covariate.
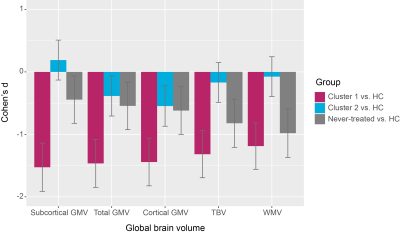
In addition to regional subcortical abnormalities, we also found that these two clusters of treated patients were different in patterns of global cerebral volume reductions, showing a similar trend as their corresponding alterations of regional subcortical volume (Figure 3). Moreover, a group of never-treated patients not included in clustering analysis showed milder reductions of global brain volume than those in severe deficit cluster of treated patients but more serious deficits than those in moderate abnormal cluster of treated patients (Figure 3).
Conclusion
In sum, we found two subtypes of patients with schizophrenia based on regional subcortical volume, displaying different degree of regional subcortical volume, global brain volume and cognitive impairment. These findings indicate critical relationships between subcortical structures and cognition in schizophrenia, and suggest that subcortical morphological abnormalities could help to capture cognitive profiles in schizophrenia.Acknowledgements
We thank all participants and their families for the involvement of this study.References
1. Okada N, Fukunaga M, Yamashita F, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry Oct 2016;21(10):1460-1466.
2. Brandl F, Avram M, Weise B, et al. Specific Substantial Dysconnectivity in Schizophrenia: A Transdiagnostic Multimodal Meta-analysis of Resting-State Functional and Structural Magnetic Resonance Imaging Studies. Biol Psychiatry Apr 1 2019;85(7):573-583.
3. Alnaes D, Kaufmann T, van der Meer D, et al. Brain Heterogeneity in Schizophrenia and Its Association With Polygenic Risk. JAMA Psychiatry Jul 1 2019;76(7):739-748.
4. Brugger SP, Howes OD. Heterogeneity and Homogeneity of Regional Brain Structure in Schizophrenia: A Meta-analysis. JAMA Psychiatry Nov 1 2017;74(11):1104-1111.
5. Chand GB, Dwyer DB, Erus G, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain Mar 1 2020;143(3):1027-1038.
6. Sugihara G, Oishi N, Son S, Kubota M, Takahashi H, Murai T. Distinct Patterns of Cerebral Cortical Thinning in Schizophrenia: A Neuroimaging Data-Driven Approach. Schizophr Bull Jul 1 2017;43(4):900-906.
7. Pan Y, Pu W, Chen X, et al. Morphological Profiling of Schizophrenia: Cluster Analysis of MRI-Based Cortical Thickness Data. Schizophr Bull Apr 10 2020;46(3):623-632.
8. Koshiyama D, Fukunaga M, Okada N, et al. Subcortical association with memory performance in schizophrenia: a structural magnetic resonance imaging study. Transl Psychiatry Jan 10 2018;8(1):20.
9. Koshiyama D, Fukunaga M, Okada N, et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep Jan 19 2018;8(1):1183.
10. Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry Feb 2008;7(1):22-28.
11. Woodward ND, Heckers S. Brain Structure in Neuropsychologically Defined Clusters of Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull Nov 2015;41(6):1349-1359.
12. Wexler BE, Zhu H, Bell MD, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry Feb 2009;166(2):189-195.
13. Czepielewski LS, Wang L, Gama CS, Barch DM. The Relationship of Intellectual Functioning and Cognitive Performance to Brain Structure in Schizophrenia. Schizophr Bull Mar 1 2017;43(2):355-364.
14. Weinberg D, Lenroot R, Jacomb I, et al. Cognitive Subtypes of Schizophrenia Characterized by Differential Brain Volumetric Reductions and Cognitive Decline. JAMA Psychiatry Dec 1 2016;73(12):1251-1259.
15. Van Rheenen TE, Cropley V, Zalesky A, et al. Widespread Volumetric Reductions in Schizophrenia and Schizoaffective Patients Displaying Compromised Cognitive Abilities. Schizophr Bull Apr 6 2018;44(3):560-574.
16. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res Jun 1 2004;68(2-3):283-297.
Figures