0201
Cerebral hemodynamic alterations associated with an in-scanner drug trial in adults with bipolar depression
William S.H. Kim1,2, Mikaela K. Dimick3,4, Danielle Omrin4, Beverley A. Orser4,5, Benjamin I. Goldstein4,6, and Bradley J. MacIntosh1,2
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada, 4Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 5Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada, 6Department of Psychiatry, University of Toronto, Toronto, ON, Canada
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada, 4Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 5Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada, 6Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Synopsis
Multiple post-label delay (multi-PLD) arterial spin labeling (ASL) magnetic resonance imaging is one approach to monitor cerebral hemodynamic drug responses. In addition to cerebral blood flow (CBF), it is possible to map arterial transit time (ATT) and arterial cerebral blood volume (aCBV). Here, we investigate multi-PLD ASL-derived CBF, ATT, and aCBV responses to a single treatment of either: 1) nitrous oxide or 2) midazolam among adults with treatment-resistant bipolar depression. Between baseline and post-treatment timepoints, we report treatment effects on CBF change in the temporal lobe and on ATT change in the frontal and parietal lobes.
Introduction
Arterial spin labeling (ASL) is a magnetic resonance imaging (MRI) technique capable of quantifying cerebral blood flow (CBF), relying on magnetically labeled blood as an endogenous tracer. ASL is non-invasive, quantitative, and amenable to repeated within-session measures, hence chosen to monitor drug effects in clinical trials.1 ASL images are often acquired with a single post-label delay (PLD) to allow for CBF quantification.2 However, multiple PLDs (multi-PLD ASL) yield additional hemodynamic parameters, namely the arterial transit time (ATT), defined as the elapsed time for blood to travel from the labeling plane to the tissue, and the arterial cerebral blood volume (aCBV), defined as the amount of blood residing in conduit arteries.3 Whereas CBF reflects neurovascular and metabolic information, ATT and aCBV are measures that instead provide macrovascular insights, which may also be clinically useful.4,5 Past ASL-based clinical trials have focused on CBF as the primary neuroimaging outcome measure as it is an established index of brain health and metabolism. In contrast, few studies have examined ATT and aCBV drug responses. Here, we report on a proof-of-principle, double-blind randomized clinical trial investigating nitrous oxide as a treatment for treatment-resistant bipolar depression.6 Nitrous oxide is believed to modulate cerebral hemodynamics in the frontal lobe; to provide additional context and to test the use for additional ASL measures, we investigated regional changes in ATT and aCBV, concurrently with CBF.Methods
25 adults with treatment-resistant bipolar depression were consented and recruited to receive either: 1) inhaled nitrous oxide plus intravenous saline, or 2) intravenous midazolam (active comparator) plus inhaled medical air.6 Multi-PLD ASL (400 ms, 800 ms, 1200 ms, 2000 ms) imaging was acquired using pseudo-continuous ASL and a two-dimensional echo-planar imaging readout (TR/TE = 4000/5.8 ms, spatial resolution, 3.9 × 3.9 × 6 mm3, field-of-view 252 × 252 × 90 mm3, 1650 ms labelling duration, and 10 control-label pairs per PLD) at baseline and post-treatment on a Philips 3T MRI Achieva system. ASL processing included motion correction, spatial regularization, generation of control-tag difference images, fitting to a 2-compartment model, voxel-wise calibration, and registration to Montreal Neurological Institute (MNI) space using tools from the FMRIB Software Library.3,7 Calibrated CBF, ATT, and aCBV maps were then smoothed with an isotropic Gaussian kernel of full-width-at-half-maximum of 5 mm. Mean CBF and ATT model estimates were extracted from the four grey matter lobes (frontal, occipital, parietal, temporal) as defined by the MNI atlas. Mean aCBV was extracted from the bilateral insula, a predefined region of interest defined by the Harvard-Oxford Atlas. We tested for a treatment effect on changes in regional CBF, ATT, and aCBV by performing Mann-Whitney’s U test on all regions separately, with an unadjusted significance level of p < 0.05.Results
12 participants received nitrous oxide treatment while 13 participants received midazolam treatment. Two participants from the nitrous oxide treatment arm were excluded (excessive motion, loss of data). Groups were matched for age and sex. Representative baseline and post-treatment CBF, ATT, and aCBV maps from a participant in the nitrous oxide treatment arm are shown in Figure 1. The time between baseline and post-treatment timepoints was 60 ± 6 minutes. Relative to nitrous oxide, midazolam treatment led to significantly greater CBF reduction in the temporal lobe (p = 0.010) (Figure 2A). Moderate, but non-significant group differences were found in the frontal (p = 0.168), parietal (p = 0.056), or occipital (p = 0.168) lobes. Meanwhile, nitrous oxide was associated with significantly greater ATT reduction in frontal (p = 0.034) and parietal lobes (p = 0.029) (Figure 2B). No group differences were found in the occipital (p = 0.293) or temporal lobes (p = 0.488). Finally, no group difference was found in the change in bilateral insula aCBV (p = 0.201) (Figure 2C).Discussion
Multi-PLD ASL demonstrated significant treatment effects on CBF and ATT change following nitrous oxide or midazolam treatment in a cohort of adults with treatment-resistant bipolar depression. These findings provide evidence that nitrous oxide is capable of engaging cerebral hemodynamics in frontal regions, in line with the overarching aim of this randomized clinical trial. Future ASL-based drug trials may benefit from multi-PLD ASL to extract complementary information to further probe drug perfusion effects.Acknowledgements
The authors wish to thank all study participants, anesthesiologists, respiratory therapists, and imaging staff for their contributions to this study.References
- Wang DJJ, Chen Y, Fernández-Seara MA, Detre JA. Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2011;337(2):359-366.
- Alsop DC, Detre JA, Golay X, et al. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magn Reson Med. 2015;73(1):106-116.
- Chappell MA, MacIntosh BJ, Donahue MJ, Günther M, Jezzard P, Woolrich MW. Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. Magn Reson Med. 2010;63(5):1357-1365.
- Hua J, Liu P, Kim T, et al. MRI techniques to measure arterial and venous cerebral blood volume. Neuroimage. 2019;187:17-31.
- MacIntosh BJ, Swardfager W, Robertson AD, et al. Regional cerebral arterial transit time hemodynamics correlate with vascular risk factors and cognitive function in men with coronary artery disease. Am J Neuroradiol. 2015;36(2):295-301.
- Dimick MK, Omrin D, MacIntosh BJ, et al. Nitrous oxide as a putative novel dual-mechanism treatment for bipolar depression: Proof-of-concept study design and methodology. Contemp Clin Trials Commun. 2020;19:100600.
- Groves AR, Chappell MA, Woolrich MW. Combined spatial and non-spatial prior for inference on MRI time-series. NeuroImage. 2009;45(3):795-809.
Figures
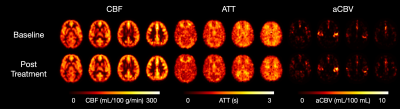
Figure 1. Representative CBF (left), ATT (middle), and aCBV (right) maps from a single participant in the nitrous oxide treatment arm at baseline (top row) and post-treatment (bottom row).
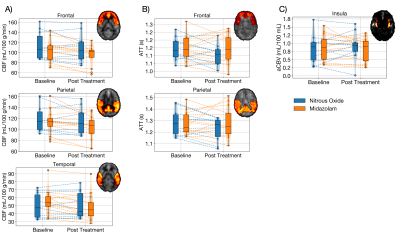
Figure 2. Regional changes in CBF (left), ATT (middle), and aCBV (right). A) Mean CBF in the frontal, parietal, and temporal lobes. B) Mean ATT in frontal and parietal lobes. C) Mean aCBV in the bilateral insula. Dashed lines represent individual participants. Insets illustrate regions of interest.