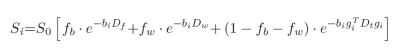0195
Three-Compartment IVIM Model Applied to Psychotic Spectrum Disorders1New York University School of Medicine, New York, NY, United States
Synopsis
We applied a three compartment intravoxel incoherent motion (IVIM) technique that estimated the perfusion fraction (PF), free water (FW), and anisotropic diffusion of tissue (FAt) to detect microvascular and microstructural changes in a cohort of 54 psychotic spectrum disorder (PSD) patients compared to 35 healthy controls (HC). We found significantly increased PF, FW and FAt in PSD and PSD subtypes compared to HC, primarily in the frontal and temporal lobes and cingulate and insular cortices at multiple comparisons correction level. In patients, IVIM metrics were found to be associated with the duration of psychosis and performance on several cognitive tests.
Background
Previous research has suggested both perfusion and free water (FW) alterations in Psychotic Spectrum Disorders (PSD)1–4, assessed independently of each other. An excess in FW, in particular, is established in PSD literature, and appears to change with disease progression5,6. Perfusion abnormalities were also reported based on DSC or ASL imaging7,8. However, the intravoxel incoherent motion (IVIM) method is a promising noninvasive technique that has been successfully applied to capture brain perfusion pathologies in several brain disorders and can be further modelled to include a FW and an anisotropic diffusion tissue compartment9,10. The three compartment IVIM-FWI model is especially promising as it disentangles FW diffusion and perfusion. The estimation of each of these two metrics may be affected when the effects of the other are not taken into consideration10. Previous histological studies have suggested an array of microvascular and microstructural deficits likely to impact perfusion and FW in PSD, including increased inflammation, morphological differences in capillaries, and disruptions in the neurovascular unit cells and the blood brain barrier11. The aim of this research is to evaluate, for the first time, if the three compartment IVIM-FWI model can describe microvascular and microstructural changes in PSD in both gray (GM) and white matter (WM). Additionally, we examined the relationships between the IVIM-FWI derived measures of perfusion fraction (PF), FW, and fractional anisotropy of tissue (FAt) and psychosis duration and cognition, which may inform upon the biological bases of PSD neuropathology and ultimately contribute to development of targeted treatment approaches.Methods
Anatomical and diffusion MRI data were acquired for 54 PSD patients (16 schizophrenia, 20 schizoaffective, 18 bipolar with psychotic features), and 35 healthy comparison controls (HC) (men and women, 18-31 years old) using a 3T Prisma MRI scanner. A three-compartment IVIM-FWI model was implemented using DMIPY software8 to obtain FW, PF, and FAt metrics (Figure 1)10,12. Mean gray and white matter FW, PF, and FAt were subsequently calculated bilaterally for the 4 cerebral GM lobes, sub-cortical WM lobes, and 34 cortical regions of interest (ROI) delineated by the Desikan-Killiany atlas1. Independent t-test analyses and analyses of covariance that accounted for age, sex, pre-morbid IQ and cortical thickness were used to evaluate differences in IVIM-FWI metrics between PSD, PSD subtypes and HC participants in each ROI. Further analyses used Pearson’s and Spearman’s correlations to test whether IVIM metrics relate to the duration from psychosis onset and cognitive function, which was assessed using a battery of working and episodic memory and processing speed neuropsychological tests. The Benjamini-Hochberg (BH) procedure was employed in each analysis to correct for multiple comparisons and decrease the false discovery rate (FDR)14. Differences were considered significant for q < .05 BH FDR and at trend-level for p < .05.Results
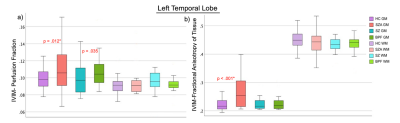
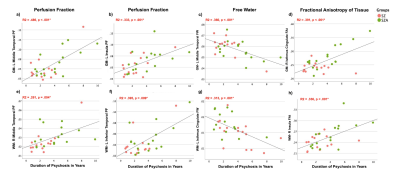
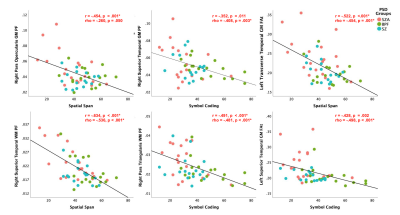
Controlling for age, sex, pre-morbid IQ and cortical thickness had no effect on group differences and therefore t-test results are presented here. Significant between-group differences were observed with: 1) significantly increased PF and FAt in GM lobar regions in PSD and PSD subtypes, and 2) significantly increased PF, FAt and FW in a number of GM and WM sub-cortical ROIs situated primarily in the frontal and temporal lobes and insular cortices in PSD and PSD subtypes (Figures 2 and 3). Significant positive relationships between PF and FAt with the duration of psychosis and significant negative relationships between FW and duration of psychosis were found in the schizophrenia spectrum disorder (SZA and SZ) subgroup in overlapping ROIs located primarily in the frontal and temporal lobes and insular and cingulate cortices (Figure 4). Furthermore, in patients but not in HC, increased PF in GM and WM and increased FAt in GM were significantly related to poor performance on several cognitive tests of memory and processing speed in the PSD group (Figure 5).Discussion
Prior IVIM studies have documented the ability of this technique to quantify brain perfusion pathologies in neurological and neurovascular diseases, but until now IVIM has not been applied in PSD populations9. Results presented here suggest that a three compartment IVIM-FWI model, which disentangles the perfusion effects from free water, can be applied to quantify both perfusion and microstructural abnormalities in PSD. In addition, these microstructural and microvascular changes appear to progress with the duration of psychosis and be implicated in healthy memory and processing speed functions. The increased PF and FW in PSD and PSD subtypes may be due to alterations in neurovascular unit cells (microglia, astrocytes, pericytes and endothelial cells), increased inflammation, morphological differences in capillaries, and degeneration of the blood brain barrier11. The increased FAt in PSD, specifically in GM, may be associated with increased glial proteins, which were noted to underlie increased FA in GM in other disorders15.Conclusion
We demonstrate, for the first time, to the best of our knowledge, that the three compartment IVIM-FWI model can be applied to PSD to quantify abnormalities in perfusion, free water and anisotropy of tissue, and that these changes appear to progress with the duration of psychosis over the first decade from onset and affect healthy memory and processing speed. IVIM-FWI metrics may provide useful biomarkers of abnormal vasculature and microstructure and help elucidate neuropathology in psychiatric and neurological disorders.Acknowledgements
This work was supported by the R01 MH108962 National Institute of Mental Health award. We greatly thank all of our participants for their help with this study and Researchmatch for supporting our recruitment effortsReferences
1. Allen, J. W. et al. Patients with Mild Cognitive Impairment May be Stratified by Advanced Diffusion Metrics and Neurocognitive Testing. J. Neuroimaging (2019).
2. Kraguljac, N. V. et al. A longitudinal neurite and free water imaging study in patients with a schizophrenia spectrum disorder. Neuropsychopharmacology (2019).
3. Lyall, A. et al. Free water imaging along the psychosis spectrum. Neuropsychopharmacology (2016).
4. Stegmayer, K. et al. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr. Res. (2017).
5. Guo, J. Y. et al. Brain free water alterations in first-episode psychosis: A longitudinal analysis of diagnosis, course of illness, and medication effects. Psychol. Med. (2019).
6. Pasternak, O., Kubicki, M. & Shenton, M. E. In vivo imaging of neuroinflammation in schizophrenia. Schizophrenia Research (2016).
7. Modinos, G. et al. Increased resting perfusion of the hippocampus in high positive schizotypy: A pseudocontinuous arterial spin labeling study. Hum. Brain Mapp. (2018).
8. Talati, P., Rane, S., Skinner, J., Gore, J. & Heckers, S. Increased hippocampal blood volume and normal blood flow in schizophrenia. Psychiatry Res. - Neuroimaging (2015).
9. Paschoal, A. M., Leoni, R. F., dos Santos, A. C. & Paiva, F. F. Intravoxel incoherent motion MRI in neurological and cerebrovascular diseases. NeuroImage: Clinical (2018).
10. Rydhög, A. S. et al. Separating blood and water: Perfusion and free water elimination from diffusion MRI in the human brain. Neuroimage (2017).
11. Kealy, J., Greene, C. & Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neuroscience Letters (2020).
12. Fick, R. H. J., Wassermann, D. & Deriche, R. The Dmipy Toolbox: Diffusion MRI Multi-Compartment Modeling and Microstructure Recovery Made Easy. Front. Neuroinform. (2019).
13. Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
14. Hochberg, B. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 57, 289–300 (1995).
15. Budde, M. D., Janes, L., Gold, E., Turtzo, L. C. & Frank, J. A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain 134, 2248–2260 (2011).
16. Vieni, C. et al. Effect of intravoxel incoherent motion on diffusion parameters in normal brain. Neuroimage (2020).
Figures