0194
3D Multi-Contrast Blood Imaging with a Single Acquisition: Simultaneous Non-Contrast-Enhanced MRA and Vessel Wall imaging1Karatsu Red Cross Hospital, Saga, Japan, 2Philips Japan, Tokyo, Japan
Synopsis
Assessment of vessel lumen and wall and plaques lesions is important in management of patients with atherosclerosis. Conventional MR imaging typically requires long scan times to acquire MRA and vessel wall imaging (VWI), respectively. In this work, we present a new multi-contrast blood imaging method named BRIDGE (bright and dark blood images with multi-shot gradient-echo-planar imaging), which simultaneously acquires MRA and VWI in a single acquisition. Initial results with volunteers and patients showed that comparable image quality to conventional methods could be acquired in a short time, allowed simultaneous assessment of luminal changes and vulnerable plaques in a single acquisition.
Introduction
Aortic vulnerable plaques are an important risk factor for systemic embolism such as cerebral infarction and myocardial infarction and peripheral embolism1. In particular, vulnerable plaques in the thoracic aorta have been found to pose a substantial risk of cerebral embolization during surgery requiring cardiopulmonary bypass or during carotid artery stenting (CAS)2,3.The 3D balanced steady-state free precession (b-SSFP)4,5 and 3D turbo field-echo (TFE)6 methods have been proposed for the conventional assessment of thoracic aortic vascular anatomy. Recently, a method using 3D multi-shot gradient-echo-planar imaging (MSG-EPI) has been proposed and is suggested to be a promising method that provides a more uniform image quality in a shorter acquisition time compared to b-SSFP7.
On the other hand, vessel wall imaging (VWI) using 3D turbo spin-echo (TSE) with variable refocusing flip angles (VRFA)8 is one of the most frequently used methods to assess vulnerable plaques of the thoracic aorta.
Most importantly, the total scan time of conventional methods is long because both MRA and VWI images are acquired in separate acquisitions.
We proposed a new method of multi-contrast blood imaging that allows obtaining both MRA and VWI images in a single acquisition using 3D MSG-EPI. In this study, we evaluated the image quality of MRA and VWI in the thoracic aorta using a new method named BRIDGE (bright and dark blood images with multi-shot gradient echo planar imaging).
Methods
BRIDGE sequence:A schematic diagram of BRIDGE sequence is shown in Figure 1. BRIDGE consists of 3D MSG-EPI acquisition and magnetization preparation with T2-prep pulse and non-selective inversion recovery (IR) pulse [Fig.1A]. Also, water excitation technique is used to suppress fat signal.
First, a T2 prep pulse consisting of four refocusing pulses was applied to reduce signals from tissues with short T2. Immediately after that, a nonselective IR pulse was applied and data were acquired at a short inversion time (TI), allowing us to acquire MRA images with suppressed background tissues with short to moderate T1 and T2, such as muscle9. Furthermore, dark blood images with suppressed blood signal could be acquired by acquiring data at a long TI10.
An important aspect of BRIDGE is to share these magnetization preparation pulses between multiple phases of the cardiac cycle in order to acquire multi-contrast blood images [Fig.1B].
Experiments:
10 healthy volunteers and 5 patients were examined on a 3.0T MR scanner (Ingenia, Philips Healthcare). BRIDGE and conventional methods - non-contrast MRA based on 3D TFE and VWI based on 3D VRFA TSE (T1-VISTA) - were performed. The study was approved by the Karatsu Red Cross Hospital Institutional Review Board and all subjects gave written informed consent.
(1) Evaluation of MRA images by BRIDGE and TFE
First, Circular regions of interest (ROIs) were placed on the vascular lumen (5 points) and background muscle to measure the mean (mean SI) and standard deviation (SD) of the signal intensity [Fig.2A]. Then, SNR and CNR were calculated using mean SI and SD of all vessels and muscles.
(2) Evaluation of black blood images by BRIDGE and T1-VISTA
The ROIs used in (1) were copied to the same locations on black blood images of BRIDGE and T1-VISTA [Fig.2B]. CNR were calculated using mean SI and SD of all vessels and muscles. In addition, to assess the uniformity of blood signal suppression, the coefficient of variation (CV) defined as SD/mean signal value of 5-point ROIs of the thoracic aorta was calculated. Finally, the image qualities of their respective images were visually assessed and compared by radiologists.
Imaging parameters for BRIDGE were; Oblique sagittal plane, FOV=320×320mm2, voxel size (ACQ) =1.6×1.6×3.0mm3, voxel size (Recon) =0.8×0.8×1.5mm3, Number of slices=50, TR=12ms, TE=5.2ms, flip angle=15°, NSA=2, EPI factor=7, TFE factor=6−8, SENSE-reduction factor=2, acquisition time (heart rate=60 beats/min) = 3m45s.
Results and Discussion
The image qualities of bright-blood and black-blood of BRIDGE were comparable to those of conventional TFE-MRA and T1-VISTA, respectively [Fig.2C,D].Representative images of these imaging techniques are shown in Figure 3.
BRIDGE allows all images of each phase to be assessed in multiple planes by MPR processing. In MIP processing, a vulnerable plaques-weighted MRA image can be produced for the simultaneous assessment of vascular anatomy and plaque location [Fig.4].
A clinical case of a patient with aortic atherosclerosis demonstrated that in BRIDGE, bright blood images may clearly visualize luminal stenosis and black blood images may clearly visualize multiple vulnerable plaques [Fig.5].
Although several methods for simultaneous imaging of MRA and VWI have recently been reported11-13, it is noteworthy that BRIDGE produces multi-contrast blood images with a single acquisition in a relatively short time, without optimal TI settings or special image processing.
Furthermore, gray blood images may identify superficial and deep calcification in atherosclerotic plaques14. With BRIDGE, gray blood images can be acquired without extended time.
Conclusion
BRIDGE provides multi-contrast blood images with a single acquisition in a relatively short time and holds promise for the assessment of systemic atherosclerosis. Further clinical studies in comparison with other state-of-the-art methods11-13 are necessary in the future.Acknowledgements
No acknowledgement found.
References
1. The French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996; 334:1216-1221
2. Szikra P, Boda K, Rarosi F, et al. Aortic arch and common carotid artery plaques with soft components pose a substantial risk of cerebral embolization during carotid stenting. Interv Neuroradiol. 2016; 22:438–444.
3. Tunick PA, Krinsky GA, Lee VS, Kronzon I. Diagnostic imaging of thoracic aortic atherosclerosis. AJR Am J Roentgenol. 2000; 174:1119-25.
4. Amano Y, Takahama K, Kumita S. Non–contrast-enhanced MR angiography of the thoracic aorta using cardiac and navigator‐gated magnetization-prepared three-dimensional steady-state free precession. J Magn Reson Imaging 2008; 27:504–509
5. Srichai MB, Kim S, Axel L, Babb J, Hecht EM. Non-gadolinium-enhanced 3-dimensional magnetic resonance angiography for the evaluation of thoracic aortic disease. A preliminary experience. Tex Heart Inst J. 2010; 37: 58–65.
6. Summers RM, Sostman HD, Spritzer CE, Fidler JL. Fast spoiled gradient-recalled MR imaging of thoracic aortic dissection: Preliminary clinical experience at 1.5 T. Magn Reson Imaging 1996; 14:1-9
7. Iyama Y, Nakaura T, Nagayama Y, et al. Comparison between multi-shot gradient echo EPI and balanced SSFP in unenhanced 3T MRA of thoracic aorta in healthy volunteers. Eur J Radiol. 2017;96:85-90.
8. Eikendal ALM, Blomberg BA, Haaring C, et al. 3D black blood VISTA vessel wall cardiovascular magnetic resonance of the thoracic aorta wall in young, healthy adults: reproducibility and implications for efficacy trial sample sizes: a cross-sectional study. J Cardiovasc Magn Reson. 2016; 18: 20.
9. Yoneyama M, Zhang S, Hu HH, et al. Free-breathing non-contrast-enhanced flow-independent MR angiography using magnetization-prepared 3D non-balanced dual-echo Dixon method: A feasibility study at 3 Tesla. Magn Reson Imaging. 2019; 63:137-146.
10. Yamada N, Higashi M, Otsubo R, et al. Association between signal hyperintensity on T1- weighted MR imaging of carotid plaques and ipsilateral ischemic events. AJNR Am J Neuroradiol. 2007; 28:287-292.
11. Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013; 69:337–345.
12. Shaw JL, Christodoulou AG, Hu Z, et al. ECG- and navigator-free 3D multi-contrast aortic vessel imaging with MR multitasking. Proceedings of the 27th annual meeting Intl Soc Mag Reson Med (ISMRM), Montreal, Canada. 2019. p. 984.
13. Yoneyama M, Zhang S, Goto Y, et al. REACT-MD: simultaneous non-contrast-enhanced subclavian MRA and fat suppressed direct thrombus imaging (MPRAGE) with a large field-of-view. Proceedings of the 28th annual meeting Intl Soc Mag Reson Med (ISMRM), Virtual Conference. 2020. p. 1320.
14. Koktzoglou I. Gray blood magnetic resonance for carotid wall imaging and visualization of deep-seated and superficial vascular calcifications.Magn Reson Med. 2013; 70:75–85.
Figures
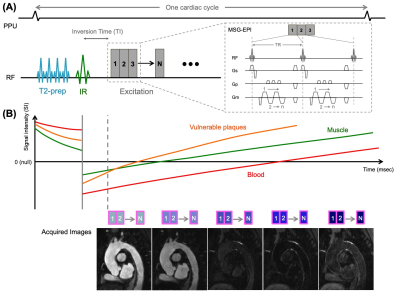
Figure 1. The schema diagram of BRIDGE with pulse gating.
BRIDGE consisting of 3D MSG-EPI with T2prep and IR pulses (A).
The estimated signal transitions of different tissue types in the thoracic aorta (B) show that data acquisition after a short inversion time (TI) yields bright blood (MRA) with suppressed background tissues such as muscle, and data acquisition after a longer TI yields dark blood images with suppressed vascular signals and enhanced vulnerable plaques.
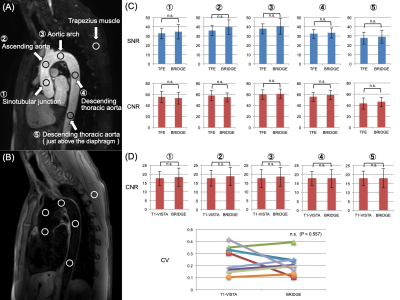
Figure 2. Comparison of BRIDGE and conventional methods.
(A) To calculate SNR and CNR, circular ROIs were placed over the vessel lumen (5 points) and background muscle on TFE MRA and BRIDGE (bright blood) images.
(B) To calculate CNR and CV, ROIs were copied to the same location on T1-VISTA and BRIDGE (black blood) images.
(C) Results of the comparison between TFE MRA and BRIDGE (bright blood).There was no significant difference in SNR and CNR.
(D) Results of the comparison between T1-VISTA and BRIDGE (black blood).There was no significant difference in CNR and CV.
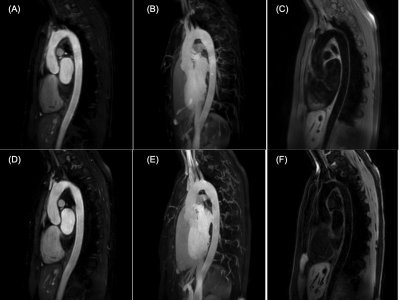
Figure 3. Representative images of conventional TFE MRA source (A) and MIP (B), T1-VISTA (C) and BRIDGE with respective bright blood source (D) and MIP (E) and black blood (F) images.
The acquisition time of each image is; (A) 2:30, (C) 3:00, (D)-(F) 3:45.
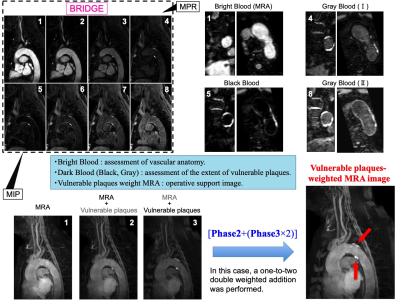
Figure 4. Optimal image processing.
All images in each phase can be assessed in multiple planes with MPR processing. Bright blood is used for assessment of vascular anatomy and vascular lumen. Dark blood such as gray or black blood is used for assessment of vessel wall and plaque characterization and the extent of vulnerable plaques.
In MIP process, creation of vulnerable plaques-weighted MRA image allows for simultaneous assessment of vascular anatomy and plaque location. This image can be used as a surgical support image such as CAS.
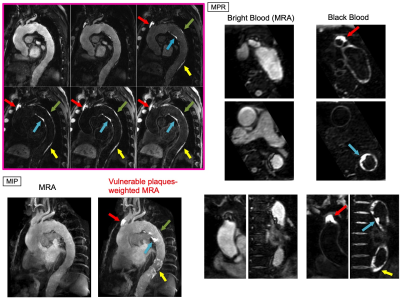
Figure 5. Representative clinical case of a patient with aortic atherosclerosis.
The source images of BRIDGE showed a vulnerable plaque at the root of the brachiocephalic artery (red arrow), and multiple plaques were clearly delineated in the thoracic aorta (blue arrow, green arrow, and yellow arrow).
On transverse and coronal MPR images, bright blood images showed intraluminal stenosis and black blood images showed a high signal strongly suggestive of the presence of vulnerable plaques.