0193
The Heterogeneity of Intramural Hematoma is Associated with Acute Ischemic Stroke in Patients with Proven Cervical Artery Dissection1Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 2Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China, 3Department of Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 4Epidemiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 5Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
Synopsis
This study investigates the relation between the heterogeneity of intramural hematoma (IMH) on three-dimensional vessel wall MRI (3D-VWMRI) images and acute ischemic stroke in patients with proven cervical artery dissection (CAD). We found the high heterogenous IMH on 3D-VWMRI was associated with acute ischemic stroke in proven and non-occluded CAD. The heterogeneous signal of an IMH on 3D-VWMRI may indicate the asynchronous progress of CAD, possibly leading to thrombosis or hemodynamic instability, therefore results in ischemic stroke. The current finding may hold promise to better elucidate the pathophysiological mechanisms and occurrence risk of ischemic stroke for patients with CAD.
Introduction
Cervical artery dissection (CAD) is a major cause of acute ischemic stroke in young and middle-aged adults1. CAD corresponds to the presence of an intramural hematoma (IMH). The signal intensity of the IMH on three-dimensional vessel wall MRI (3D-VWMRI) varies over time and can facilitate determine the age of the dissection2. However, the heterogeneous signal of an IMH on VWMRI may indicate the asynchronous progress of CAD, possibly leading to thrombosis or hemodynamic instability, therefore results in ischemic stroke. We aimed to investigate the association between the heterogeneity of IMH on 3D-VWMRI and acute ischemic stroke in patients with proven CAD.Methods
Our study sample was based on the prospective population of Whole Brain Vessel Wall Imaging in Stroke Patients (WISP) study. The patients suspected of CAD were prospectively invited to receive the VWMRI examination. Sixty-two patients with proven CAD (mean age, 40.6 years ± 10.3; 40 men) were included in the current study and were divided into acute ischemic stroke group and non-stroke group. The segmentation of IMHs was performed using 3D-Slicer software3 with the intensity of individual sternocleidomastoid used as the threshold. The total volume of IMH (VIMH), the volume of high-intensity IMH (V1), and the volume of equal or low-intensity IMH (V2) were calculated. The heterogeneity of IMH on VWMRI was defined as the absolute value of V1 minus V2 divided by VIMH. The stenosis degree of dissected lesions, the lesion length, the presence/absence of intraluminal thrombus were also evaluated. The optimal cut-off value of heterogeneity of IMH for acute ischemic stroke was assessed using receiver operating characteristic (ROC) analysis. Multiple logistic regression was used to examine the association between the heterogeneity of IMH on 3D-VWMRI and acute ischemic stroke.Results
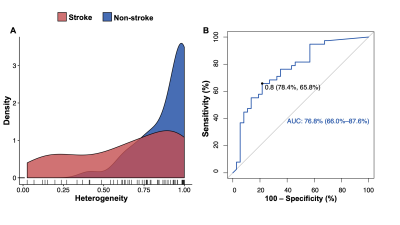
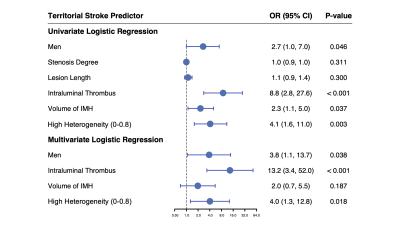
A total of 75 non-occluded arteries with IMH were analyzed. Thirty-eight of 75 (51%) dissected arteries were found with acute territory ischemic stroke, 37 of 75 (49%) without stroke. The ROC curve analysis indicated that the optimal cut-off value of the heterogeneity of IMH was 0.8. The area under the ROC curve was 0.768 (Figure 1). The IMHs with high heterogeneity (0-0.8) were more prevalent in the group of acute ischemic stroke than the group of non-stroke (60.5% vs. 27.0%, P < 0.001). In multivariable logistic regression analysis, the presence of intraluminal thrombus and high heterogenous (heterogeneity was 0-0.8) IMH on VWMRI were independently associated with acute ischemic stroke in proven CAD with odds ratios of 13.2 (95% CI: 3.2–52.0, P < 0.001) and 4.0 (95% CI, 1.3–12.8, P = 0.018), respectively (Figure 2). Figure 3 shows the representative cases with high heterogenous IMH and low heterogenous (0.8-1) IMH.Discussion
Previous studies demonstrated the specific CAD imaging risk factors of ischemic stroke on MRI including IMH, irregular surface, and intraluminal thrombus4,5. The present study has further identified that the heterogeneity of IMH on 3D-VWMRI significantly contributed to acute ischemic stroke, which adds new data over prior works. The homogenous IMH signal on 3D-VWMRI supports the one-time event of CAD. The heterogeneous 3D-VWMRI signal intensity of IMH depends on the mixing age of the CAD. Variability in intensity relates to fresh clot formation and sedimentation of cellular components in the plasma6. The heterogeneous IMH could indicate recurrent hemorrhages or an uneven speed of decomposition. In non-occluded vessels, it may more frequently cause territory ischemic stroke due to a thromboembolic mechanism or a hemodynamic mechanism7. From a clinical perspective, the dissected patients without occluded vessels but with heterogeneous IMH may therefore require a closer clinical observation than those with homogeneous IMH thus should be practical for planning future therapeutic strategies.Conclusion
The current findings indicate that the IMH with high heterogeneity and the presence of intraluminal thrombus were related to acute ischemic stroke occurrence in patients with CAD. It may hold promise to better triage patients with impending acute ischemic stroke in clinical practices associated with CAD.Acknowledgements
This work was supported in part by the National Key R&D Program of China (2017YFC1308000), the Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20180602), the Beijing Natural Science Foundation (Nos. 7191003), and the National Science Foundation of China (NSFC 8191101305).References
1. Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8(7):668-678. doi: 10.1016/S1474-4422(09)70084-52.
2. Habs M, Pfefferkorn T, Cyran CC, Grimm J, Rominger A, Hacker M, et al. Age determination of vessel wall hematoma in spontaneous cervical artery dissection: a multi-sequence 3T cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011;13:76. doi: 10.1186/1532-429X-13-763.
3.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012;30(9):1323-1341. doi: 10.1016/j.mri.2012.05.0014.
4. Wu Y, Wu F, Liu Y, Fan Z, Fisher M, Li D, et al. High-Resolution Magnetic Resonance Imaging of Cervicocranial Artery Dissection: Imaging Features Associated With Stroke. Stroke. 2019;50(11):3101-3107. doi: 10.1161/STROKEAHA.119.0263625.
5. McNally JS, Hinckley PJ, Sakata A, Eisenmenger LB, Kim SE, De Havenon AH, et al. Magnetic Resonance Imaging and Clinical Factors Associated With Ischemic Stroke in Patients Suspected of Cervical Artery Dissection. Stroke. 2018;49(10):2337-2344. doi: 10.1161/STROKEAHA.118.0218686.
6. Delcourt C, Zhang S, Arima H, Sato S, Al-Shahi Salman R, Wang X, et al. Significance of Hematoma Shape and Density in Intracerebral Hemorrhage: The Intensive Blood Pressure Reduction in Acute Intracerebral Hemorrhage Trial Study. Stroke. 2016;47(5):1227-1232. doi: 10.1161/STROKEAHA.116.0129217.
7. Morel A, Naggara O, Touze E, Raymond J, Mas JL, Meder JF, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361. doi: 10.1161/STROKEAHA.111.643338
Figures