0192
Quantitative Time-of-Flight (qTOF) and Quiescent Interval Slice-Selective (qQISS) Intracranial MR Angiography1Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 2University of Chicago Pritzker School of Medicine, Chicago, IL, United States, 3Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Synopsis
Time of flight (TOF) and quiescent interval slice selective (QISS) magnetic resonance angiography (MRA) provide accurate anatomic evaluation blood vessels but do not readily quantify blood velocity or flow. Addressing this deficiency, we report two multi-echo stack-of-stars variants of TOF and QISS MRA (which we refer to as “quantitative TOF” (qTOF) and “quantitative QISS” (qQISS) MRA) that provide for simultaneous high-resolution anatomic and hemodynamic evaluation of the intracranial arteries.
Introduction
Time-of-flight (TOF)1 and quiescent interval slice-selective (QISS)2 are accurate nonenhanced MRA techniques for diagnosing intracranial and lower extremity arterial disease, respectively. However, both methods only provide anatomic evaluation of blood vessels, and do not quantify blood velocity or flow. Inspired from recent work leveraging a thin-slab multi-echo stack-of-stars QISS protocol for portraying the neck arteries3, we report 3D stack-of-stars multi-echo variants of TOF and QISS, which we refer to as quantitative TOF (qTOF) and quantitative QISS (qQISS), that simultaneously portray and quantify blood flow velocity in the intracranial arteries. Portrayal of the intracranial arteries with respect to the brain tissue for qTOF and qQISS is achieved through time-of-flight-based saturation and/or inversion-recovery based suppression of brain tissue, while blood flow velocity, directionality, and flow rate can be quantified throughout the intracranial arteries via 3D analysis of small blood flow displacements between echo times.Methods
This study was approved by our institutional review board and all participants (n=8, 4 male, age range=22-61 years) provided written informed consent. Imaging was done on a 3T MRI system (MAGNETOM Skyrafit, Siemens Healthineers, Erlangen) equipped with a 12-element head coil. Nonenhanced 3D qTOF and qQISS MRA of the circle of Willis was done over 5.2cm of head-foot coverage with the following parameters: 260×260mm2 field of view, 0.58×0.58×1.0mm3 spatial resolution reconstructed to 0.29×0.29×0.5mm3, fast low-angle shot readout with echo times of 2.9-3.4ms (TE1), 5.1-5.6ms (TE2), and 7.2-7.8ms (TE3), 213 radial views, qTOF/qQISS inter-echo TR of 21.1/13.1ms, flip angle 15°, QISS inversion-recovery period of 1500ms with a TI of 1000ms, 3 axial slabs with 8 mm overlap, scan time of 4min 3s, tilted optimized non-saturating (i.e. TONE) RF excitation, bandwidth 587Hz/pixel, flow compensation of TE1 and TE3.High-resolution MR angiograms were constructed using the TE1 data, as well as by root-mean-square combination3 of data acquired at TE1 and TE3 to improve signal-to-noise ratio (SNR). Arterial blood flow displacement between TE1 and TE3 was quantified in 3D using a custom computational framework leveraging center-of-mass tracking and template matching. Blood flow velocity was computed on a pixel-by-pixel basis as flow displacement (in distance units) divided by the time elapsed between TE1 and TE3, followed by local smoothing.
For evaluation of arterial anatomy, qTOF and qQISS were compared to resolution- and scan-time-matched conventional Cartesian 3D TOF MRA (TR/TE=21.0ms/3.4ms, bandwidth 186Hz/pixel), and to resolution-matched 3D phase-contrast (PC) MRA (TR/TE=47.8ms/7.3ms, flip angle 10°, venc=60cm/s, bandwidth 440Hz/pixel, scan time 4min 40s). Mean cross-sectional blood velocities obtained in the middle and posterior cerebral arteries (bilateral M1, M2, P2 segments) with qQISS and qTOF were compared to values obtained with 3D PC MRA. In light of the low SNR of the resolution-matched 3D PC protocol, 3D PC was also acquired at the lower resolution of 1.2×1.2×1.3mm3 (reconstructed to 0.6×0.6×0.65mm3).
Results
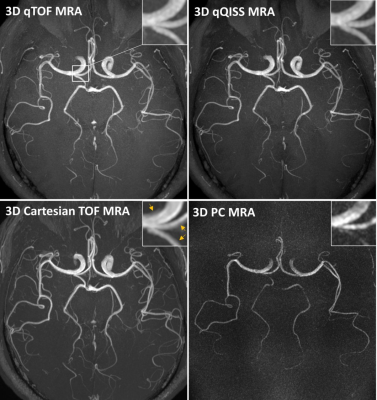
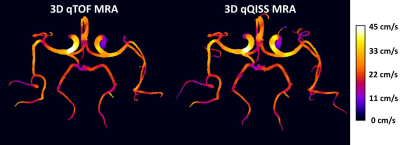
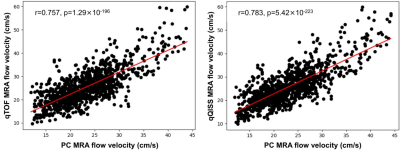
Figure 1 shows transversal maximum intensity projections (MIP) images obtained with the multi-echo stack-of-stars qTOF and qQISS techniques with respect to resolution-matched and scan time-matched standard-of-care Cartesian TOF and 3D PC MRA techniques. Excellent anatomical correlation of qTOF and qQISS MRA was found with respect to conventional Cartesian TOF MRA, and both protocols provided superior SNR and arterial delineation with respect to resolution-matched 3D PC MRA. Stack-of-stars qTOF and qQISS also eliminated flow misregistration (i.e., signal “pileup”) artifact seen with conventional Cartesian TOF MRA on transversal MIP images. Figure 2 shows examples of blood velocity maps provided by the qTOF and qQISS techniques. Figure 3 shows the correlation of mean cross-sectional blood flow velocity in the middle and posterior cerebral arteries between qTOF and qQISS and lower resolution 3D PC MRA. Strong positive correlations (r=0.757 for qTOF, P=1.29×10-196; r=0.783 for qQISS, P=5.42×10-223) with respect to 3D PC MRA were found.Discussion
We introduced two novel multi-echo stack-of-stars based MRA techniques – “qTOF” and “qQISS” – along with a novel computational framework that allows for high spatial resolution display of the intracranial arteries and flow quantification in visualized arterial segments. Excellent anatomical correlation of the intracranial arteries with both stack-of-stars qTOF and qQISS was found with respect to conventional Cartesian TOF MRA, and strong positive correlations for mean cross-sectional blood flow velocity were found with respect to 3D PC MRA. qTOF and qQISS provided substantially higher SNR than resolution-matched 3D PC MRA, while the use of a stack-of-stars acquisition reduced flow misregistration artifact4 seen with conventional Cartesian TOF MRA. Further improvements in arterial-to-background contrast are anticipated with the application of magnetization transfer contrast.Conclusion
In conclusion, qTOF and qQISS represent new quantitative MRA methods that provide for high spatial resolution anatomic evaluation of the intracranial arteries while allowing for simultaneous quantification of blood flow in all visualized arteries. The techniques hold promise for high-resolution anatomic and hemodynamic evaluation of the intracranial vessels in a single time-efficient acquisition.Acknowledgements
FUNDING SOURCE: NIH grant R01 EB027475References
1. Parker DL, et al. Magn Reson Med. 1991 Feb;17(2):434-51. doi: 10.1002/mrm.1910170215.
2. Edelman RR, et al. Magn Reson Med. 2010 Apr;63(4):951-8. doi: 10.1002/mrm.22287.
3. Koktzoglou I, et al. Magn Reson Med. 2020 Dec;84(6):3316-3324. doi: 10.1002/mrm.28339.
4. Nishimura DG, et al. Magn Reson Med. 1991 Dec;22(2):481-92. doi: 10.1002/mrm.1910220255.
Figures