0190
Gadolinium-free Multi-contrast 3D whole-heart MRI for improved anatomical assessment in patients with Congenital Heart Disease1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Adult Congenital Heart Disease Department, Guy’s and St Thomas’s Hospital, London, United Kingdom
Synopsis
Bright- and black-blood MRI sequences provide complementary information on anatomical assessment for patients with Congenital Heart Disease(CHD).A free-breathing 3D whole-heart sequence (MTC-BOOST) has been recently proposed for simultaneous bright and black-blood high quality depiction of cardiac and vascular structures waiving the need for contrast agent injection. In this study, we clinically validated this sequence in a cohort of 35 patients with CHD against the conventional T2-prep bSSFP sequence. Quantitative and qualitative image quality assessment demonstrates that MTC-BOOST enhances pulmonary venous depiction, mitigates flow-related artefacts and has comparable image quality for intracardiac and vascular structures, promising potential integration into clinical workflow.
Introduction
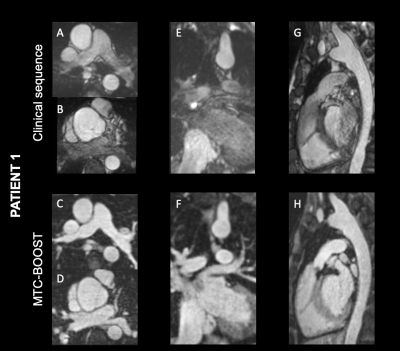
Bright- and black-blood whole heart (WH) imaging sequences are utilised for the morphological evaluation of patients with Congenital Heart Disease (CHD). Current clinical approaches commonly include a 2D breath-hold Half-Fourier Acquisition Single-shot Turbo spin Echo sequence (HASTE)1 for black-blood visualisation along with a 3D T2-prepared balanced Steady State Free Precession (T2-prep bSSFP)1 bright-blood sequence. Limitations include patient discomfort due to multiple breath-holds and 2D anatomic visualisation with the HASTE sequence, along with long scan times due to diaphragmatic navigators2 as well as flow and off-resonance related image artefacts3,4 for the T2prep-bSSFP sequence. Gadolinium based contrast agents are often employed to enhance the image quality of T2prep-bSSFP, albeit with associated side effects5. We have recently proposed a sequence that enables simultaneous acquisition of 3D Bright- and black-blOOd phase SensiTive (BOOST)6 motion compensated images under free-breathing; and have advanced this by exploiting Magnetisation Transfer Contrast (MTC-BOOST) (Figure 1A&B) for high quality depiction of both the arterial and the venous systems without the need of contrast agent injection. The use of 2D image-based navigation7 enables beat-to-beat translational and bin-to-bin non-rigid respiratory motion and 100% respiratory scan efficiency. The undersampled motion-corrected reconstruction framework is implemented in-line in the scanner software. The aim of this study is to validate the proposed sequence against the clinical T2-prep bSSFP with respect to the visualisation of intracardiac and vascular structures.Methods
Data acquisition: MTC-BOOST was acquired in 35 patients (33 years old ± 12, 23 Male) with CHD on a 1.5T system (MAGNETOM Aera, Siemens Healthcare). Imaging parameters included: free-breathing no respiratory gating, subject specific mid-diastolic ECG-triggering, coronal orientation, resolution 1.5mm3 isotropic, flip angle 90°, TE/TR 1.35/3.2ms, undersampling of 3-fold, MT preparation:15 Gaussian pulses, flip angle 800, frequency offset 3000Hz, duration 20.5ms.Data analysis: The T2-prep bSSFP 3D WH dataset acquired as part of the clinical protocol was compared against the research MTC-BOOST dataset. A cardiologist blinded to the acquisition method assessed the luminal signal, expressed as lumen/myocardium contrast ratio for cardiac and vascular structures. Additionally relevant image quality scores for nine vascular structures using 5-point Likert scale ( 3≥ diagnostic image) and co-axial aortic dimensions at the level of the proximal ascending aorta, obtained with both methods, were evaluated by the same blinded reader for comparison.
Results
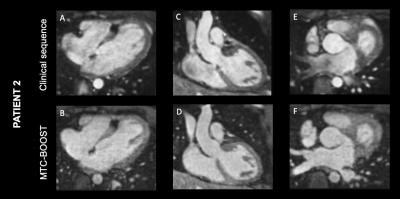
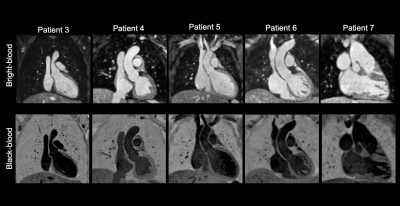
Bland-Altman plot demonstrated excellent agreement of aortic dimensions assessment at the level of the proximal ascending aorta between the MTC-BOOST and the clinical sequence (Figure 2A). Contrast ratio (CR) for the pulmonary veins was significantly higher for the MTC-BOOST sequence (Mann Whitney U test, p <0.05, Figure 2B) and the CR was sufficiently diagnostic (>1) for all the intra-pericardiac vascular structures. Image quality scores for the assessed vascular structures were persistently high in the MTC-BOOST dataset (p<0.05 Wilcoxon signed rank test, Figure 2C) with marked superiority of the MTC-BOOST sequence in the delineation of pulmonary veins. MTC-BOOST resulted in improved luminal signal and was robust towards flow alterations and off-resonance effects (Figure 3 and 4). Consistent good umage quality was observed for both bright- and black-blood images for patients with different diagnoses (Figure 5).Discussion
The proposed MTC-BOOST sequence offers improved depiction of the systemic and pulmonary venous systems compared to the conventional approach along with good delineation of the intracardiac and arterial anatomy that is comparable to the clinical T2prep bSSFP. Flow artefacts and off-resonance artefacts are attenuated with the proposed approach.Conclusion
MTC-BOOST provided high quality, non-contrast, 3D bright- and black-blood visualization of the cardiac and vascular anatomy in a cohort of patients with CHD. The proposed method may obviate the need for gadolinium-based contrast agents in this patient group and promises to be beneficial in clinical practice.Summary of main findings
A free-breathing contrast-free 3D whole-heart sequence, MTC-BOOST, for simultaneous bright- and black-blood visualisation has been validated in a cohort of 35 patients with CHD, demonstrating improved image quality and promising potential integration in clinical settings.Acknowledgements
The authors acknowledge financial support from the BHF PG/18/59/33955, EPSRC EP/P001009/, EP/P032311/1, EPSRC EP/P007619, Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1), and the Department of health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St. Thomas’ NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.References
1. Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. Journal of cardiovascular magnetic resonance. 2013;15(1):51. doi: 10.1186/1532-429X-15-51.
2. Greil G, Tandon AA, Silva Vieira M, Hussain T. 3D whole heart imaging for congenital heart disease. Frontiers in pediatrics. 2017;5:36. https://www.ncbi.nlm.nih.gov/pubmed/28289674. doi: 10.3389/fped.2017.00036.
3. Markl M, Alley MT, Elkins CJ, Pelc NJ. Flow effects in balanced steady state free precession imaging. Magnetic resonance in medicine. 2003;50(5):892-903. https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.10631. doi: 10.1002/mrm.10631.
4. Hu P, Stoeck CT, Smink J, et al. Non-contrast SSFP pulmonary vein MRA: Impact of off-resonance and flow. Journal of magnetic resonance imaging. 2010;32(5):1255-1261. https://explore.openaire.eu/search/publication?articleId=od267::198e7bc128eedb8788f70e011bd3814f. doi: 10.1002/jmri.22356.
5. Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals. 2016;29(3):365-376. https://search.datacite.org/works/10.1007/s10534-016-9931-7. doi: 10.1007/s10534-016-9931-7.
6. Ginami, G., Lòpez, K., Mukherjee, R., Neji, R., Munoz, C., Roujol, S., Mountney, P., Razavi, R., Botnar, R. and Prieto, C., 2018. Non‐contrast enhanced simultaneous 3D whole‐heart bright‐blood pulmonary veins visualization and black‐blood quantification of atrial wall thickness. Magnetic Resonance in Medicine, 81(2), pp.1066-1079.
7. Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med. 2012;67(2):437-445. doi:10.1002/mrm.23027
Figures

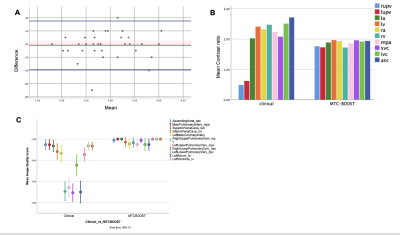
A. Bland-Altman analysis for the Ascending aorta diameter shows excellent agreement with a mean difference of 0.02 mm (95% confidence interval -0.39-to-0.35, p=0.7).
B. MTC-BOOST shows consistently high contrast ratio >1 and is comparable to the clinical sequence with significantly higher contrast ratio for the right and left upper pulmonary vein (p= 0.0001).
C. Error bars showing mean quality scores and 95% confidence intervals for nine relevant vascular structures.