0188
Focused navigation (fNAV) for cardiac and respiratory motion-compensated free-running 3D whole-heart coronary MRA
Giulia MC Rossi1, Nemanja Masala1, Jessica AM Bastiaansen1, Aurelien Bustin1,2, Jérôme Yerly1,3, John Heerfordt1,4, Davide Piccini1,4, Matthias Stuber1,3, and Christopher W Roy1
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2LIRYC (Electrophysiology and Heart Modeling Institute), Bordeaux, France, 3CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 4Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare AG, Lausanne, Switzerland
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2LIRYC (Electrophysiology and Heart Modeling Institute), Bordeaux, France, 3CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 4Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare AG, Lausanne, Switzerland
Synopsis
A novel method for reconstructing motion-compensated 3D CMRA images from free-running data is proposed. We extend the use of a recent method for non-rigid respiratory motion correction called focused navigation (fNAV) to also encompass cardiac motion compensation that accounts for beat-to-beat heart-rate variability. Our combined fNAV approach is compared to the previously established cardiac and respiratory motion-resolved 5D imaging in vivo and is shown to provide overall similar image quality and comparable right coronary artery visualizations to 5D imaging in significantly shorter reconstruction times.
Introduction
Coronary Magnetic Resonance Angiography (CMRA) requires robust compensation of both cardiac and respiratory motion. The free-running framework1-2 allows for cardiac and respiratory motion-resolved 5D imaging, where an end-expiratory mid-diastolic motion state can be retrospectively identified to effectively “freeze” motion for coronary visualization. However, unresolved intra-bin motion, heart-rate variability, and undersampling artifacts may still degrade the image quality.Recently, an auto-focusing technique (fNAV)3 was proposed for intra-acquisition non-rigid correction of respiratory motion in 3D radial ECG-triggered CMRA acquisitions. Using fNAV, high quality visualizations of the coronary arteries can be obtained by combining data from the entire respiratory cycle. Still, prospectively triggered images can suffer from incorrect resting phase calculation or heart-rate variability.
In this work, we developed a novel method for whole-heart CMRA that extends and integrates the fNAV approach with a previously published free-running1-2 uninterrupted bSSFP acquisition using LIBRE water excitation pulses4-6. We test the hypothesis that, a) by using fNAV to correct for non-rigid respiratory motion and b) by integrating a subject-specific auto-focusing strategy for retrospective cardiac soft-gating, we can obtain high-quality images of coronary arteries accounting for heart-rate variability without the need for prospective calibration scans7 or long compressed sensing (CS) reconstructions. We quantitatively compare our approach to the previously established cardiac and respiratory motion-resolved 5D imaging in vivo.
Methods
Acquisition. CMRA data were acquired in 19 healthy volunteers (age: 23-38 y; 10 male) with written informed consent on 1.5T clinical scanners (MAGNETOM Aera and MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany) with a previously described prototype free‐running acquisition7 while simultaneously recording the ECG signal.Reconstruction. For each volunteer, two reconstruction approaches were compared. First, 5D images were reconstructed using CS as previously reported1-2,6 and a 3D volume corresponding to a manually identified quiescent cardiac phase at end-expiration was selected. In the second approach, a quiescent 3D volume was obtained by correcting an automatically selected subset of quiescent cardiac data for respiratory motion using fNAV (Fig.1), according to the following steps:
- Respiratory motion amplitudes are estimated with fNAV3 and are used for rigid correction of respiratory motion in k-space.
- Quiescent cardiac data selection and soft-gating is performed by extending the auto-focusing concept to the cardiac motion model presented in [7]. Based on the ECG time stamp, for each heart-beat i, the trigger delay (Td) is computed as: $$Td(i)=k_0·T_{RR,AVG}(i)+(1-k_0)·k_1·log(10·(T_{RR,AVG}(i) +k_2))$$ where, to account for heart-rate variability, the R-R interval is computed for each heart-beat based on the average of the five preceding heart-beats. A window of empirically determined fixed width (w) starting from Td is considered to be quiescent and corresponding readouts are weighted by a Gaussian function centered at Td(i)+w/2. Here, k0 replaces an offset value traditionally determined through calibration scans7, while k1 and k2 are related to the duration of the systolic and diastolic portions of the cardiac cycle and are age and gender dependent. In our approach, the three coefficients are considered subject-specific and are iteratively estimated by evaluating the image gradient entropy of intermediate images obtained with respiratory motion correction from (1) and the current cardiac soft-gating.
- Final non-rigid respiratory motion correction of the cardiac soft-gated data from (2) is performed using fNAV3.
Results
Overall, fNAV reconstructions were significantly faster than 5D reconstructions (39±6 minutes vs 479±88 minutes, p<10-13), while achieving comparable image quality (Fig.2). Reformats of the RCA were also similar; however, the fNAV approach provided improved visual vessel conspicuity at specific locations (Fig.3). Quantitative comparisons revealed improved CR with fNAV (p<10-4), comparable visible RCA length, equal RCA detection rates and no statistically significant difference in %VS (Table1).Discussion and Conclusion
Using our fNAV approach, we were able to correct free-running whole-heart CMRA data for non-rigid respiratory motion and by extending the auto-focusing concept to retrospective cardiac soft-gating, we were able to provide subject-specific compensation of cardiac motion that accounts for heart-rate variability. This combined approach produced high-quality CMRA images that were comparable to previously established 5D image reconstructions of the same data, but with a much shorter reconstruction time. In the current work, we used fNAV to produce static 3D volumes in contrast to the dynamic 5D image reconstructions. In principle, dynamic data could be generated by the fNAV method by shifting the cardiac soft-gating window, and provided enough data remain in our temporal footprint during peak motion. Furthermore, in patient populations where un-resolved intra-bin respiratory motion and heart-rate irregularities may be further amplified and degrade image quality of 5D imaging, our approach may be particularly beneficial, but this remains to be studied. Finally, if sufficient SNR gain could be achieved whilst preserving %VS, the increased amount of signal could be traded for higher resolution or abbreviated scan times, potentially improving the current clinical utility of 3D radial CMRA.Acknowledgements
No acknowledgement found.References
- Di Sopra, L., Piccini, D., Coppo, S., Stuber, M., & Yerly, J. (2019). An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magnetic resonance in medicine, 82(6), 2118-2132.
- Feng, L. I., Coppo, S., Piccini, D., Yerly, J., Lim, R. P., Masci, P. G., ... & Otazo, R. (2018). 5D whole‐heart sparse MRI. Magnetic resonance in medicine, 79(2), 826-838.
- Roy, C. W., Heerfordt J., Piccini, D., Schwitter, J.,
& Stuber, M. Motion Compensated Coronary MRA using Focused Navigation
(fNAV). Proc. Intl. Soc. Mag.
Reson. Med. 28 1321 (2020).
- Bastiaansen, J. A., & Stuber, M. (2018). Flexible water excitation for fat‐free MRI at 3T using lipid insensitive binomial off‐resonant RF excitation (LIBRE) pulses. Magnetic resonance in medicine, 79(6), 3007-3017.
- Bastiaansen, J. A., van Heeswijk, R. B., Stuber, M., & Piccini, D. (2019). Noncontrast free-breathing respiratory self-navigated coronary artery cardiovascular magnetic resonance angiography at 3 T using lipid insensitive binomial off-resonant excitation (LIBRE). Journal of Cardiovascular Magnetic Resonance, 21(1), 38.
- Masala, N., Bastiaansen, J. A., Di Sopra, L., Roy, C. W., Piccini, D., Yerly, J., ... & Stuber, M. (2020). Free‐running 5D coronary MR angiography at 1.5 T using LIBRE water excitation pulses. Magnetic resonance in medicine, 84(3), 1470-1485.
- Roes, S. D., Korosoglou, G., Schär, M., Westenberg, J. J., Van Osch, M. J., De Roos, A., & Stuber, M. (2008). Correction for heart rate variability during 3D whole heart MR coronary angiography. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 27(5), 1046-1053.
- Etienne, A., Botnar, R. M., Van Muiswinkel, A. M., Boesiger, P., Manning, W. J., & Stuber, M. (2002). “Soap‐Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 48(4), 658-666.
- Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J. C., Pujol, S., ... & Buatti, J. (2012). 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic resonance imaging, 30(9), 1323-1341.
Figures

Figure
1. Overview of the 3-step auto-focusing framework for reconstructing cardiac
and respiratory motion-compensated 3D whole-heart CMRA images from free-running data.
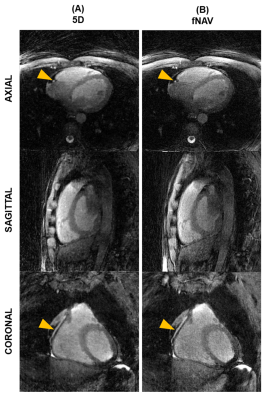
Figure 2.
Qualitative comparison between 5D and fNAV-based reconstructions. Representative images in axial, sagittal, and coronal orientations
are shown for a mid-diastolic end-expiration phase in the reference 5D
reconstruction (A) and for the proposed fNAV reconstruction (B). Yellow arrows
indicate locations where a portion of the right coronary artery is visible.
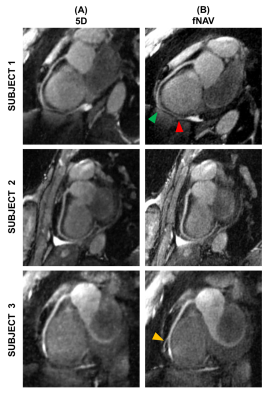
Figure 3.
Reformatted images of the right coronary artery (RCA). Reformatted images of the RCA obtained with Soap-Bubble
are shown for three representative volunteers for the reference 5D
reconstruction (A) and for the proposed fNAV-based reconstruction (B). Arrows
indicate RCA branches (yellow) or segments (green: beginning of the segment,
red: end of the segment) for which the fNAV-based reconstruction approach
allowed for improved detail visibility.
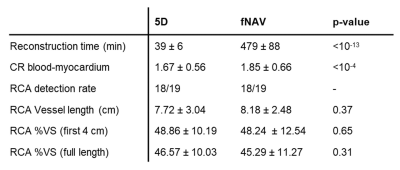
Table 1. Quantitative
comparison between 5D and fNAV-based reconstructions. CR,
contrast ratio; RCA, right coronary artery; VS, vessel sharpness. Data are
presented as mean ± SD, except for detection rates. RCA was considered
detected if >2cm were visible. Differences in reconstruction time and
blood-myocardium CR were statistically significant at a 0.05 significance level
(paired t-test).