0186
Ferumoxytol-enhanced Pulmonary MRA in Pregnancy: Evaluation of Initial Safety and Image Quality1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medicine, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Ferumoxytol (Feraheme®, AMAG Pharmaceuticals, Waltham) is an injectable iron micro particle-solution approved by the FDA for treatment of iron-deficiency anemia. This medication also has favorable properties as intravascular blood pool agent (off-label) for contrast-enhanced MRA (Fe-MRA). Fe-MRA is an alternative modality for the assessment of pulmonary embolism without the need for ionizing radiation or gadolinium in pregnant patients. The purpose of this study was to summarize our clinical experience with 70 Fe-MRA exams in 66 pregnant patients. We aimed to demonstrate the safety and efficacy of Fe-MRA and provide guidance in establishing a clinical service.
Introduction
Pulmonary embolism (PE) is a leading cause of death among pregnant patients with a mortality rate up to 30%, if untreated1. A timely and accurate diagnosis is crucial.Traditional imaging for diagnosing PE include chest radiography, computed tomographic angiography (CTA) and ventilation/perfusion lung scan1,2, exposing both mother and fetus to radiation2. While gadolinium-based contrast-enhanced MRA (GBCA-MRA) is an ionizing radiation-free alternative3–5, GBCA-MRI during pregnancy has been linked to rheumatological/inflammatory conditions in addition to stillbirth and neonatal death6.
Ferumoxytol (Feraheme®, AMAG Pharmaceuticals, Waltham) is approved for parenteral treatment of iron-deficiency anemia in chronic renal disease and during pregnancy7. Ferumoxytol is comprised of superparamagnetic iron oxide particles, and has favorable MRI properties, including excellent R1 relaxivity8 and pharmacokinetics, allowing its usage as an blood pool agent for ferumoxytol-enhanced MRA (Fe-MRA). Fe-MRA’s extended blood pool/plasma half-life allows image acquisition over an extended temporal window (e.g. free-breathing)9–11. As it has been used for vascular imaging11,12, Fe-MRA can be considered as a viable imaging modality during pregnancy. Therefore, the purpose of this study is to summarize our clinical experience with Fe-MRA in pregnant patients, demonstrate its safety, and provide guidance for clinical service.
Methods
This retrospective study was performed with IRB approval, including a waiver of informed consent. Electronic health records were searched for Fe-MRA exams performed in pregnant patients between 1/1/2017 and 11/17/2020.Workflow for ferumoxytol-enhanced pulmonary MRA
Based on literature and a 3/31/15 communication from the FDA13, our institution adopted the following guidelines: the Fe-MRA indication is submitted by clinical staff, and approved by a radiologist.
Following ISMRM guidelines for the safe use of ferumoxytol with MRI14, we use a weight-based of 3mg/kg (maximum dose 510mg), diluted in 100ml of saline, and infused over 15min (1-2mL/min), followed by 25mL saline injected (1-2mL/min). Fe-MRA contraindications include allergy to iron and systolic blood pressure <90mmHg.
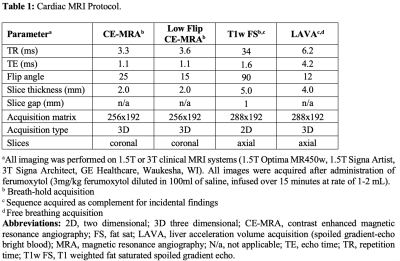
Clinical nursing staff performs standard precautions/monitoring to assess for adverse reactions during/after Ferumoxytol IV infusion. The patient’s blood pressure is monitored prior to, 5, and 30 minutes after ferumoxytol administration. Detailed Fe-MRA protocol is shown in Table 1.
Data collection and evaluation
The following data was collected: age, sex, weight, height, gestational age, ferumoxytol dose, information about adverse events (if any), Fe-MRA scan time, order time and report time; and any additional imaging performed after Fe-MRA (if any) to assess for PE.
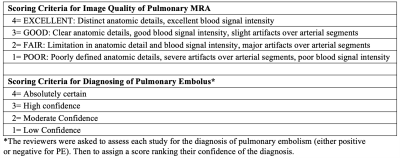
All images were reviewed independently by three cardiovascular imagers (8-30 years of experience) to tabulate abnormal MRI findings, assess image quality (scores: 4=best, 1=worst; Table 2), and score Fe-MRA exams based on reader’s confidence for the diagnosis of PE (Table 2).
Statistical analysis was performed using Excel (v16.30, Microsoft, Washington). Continuous data are presented as mean ±standard deviation. For image quality, interclass correlation (ICC) estimates were calculated using R version 4.0.3 (irr package, version 0.84.1) based on a mean-rating (k=3), absolute-agreement, 2-way mixed-effects model. The overall image quality is given as a mean of all three readers.
Results
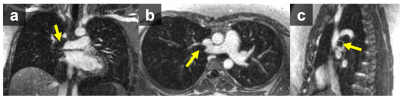
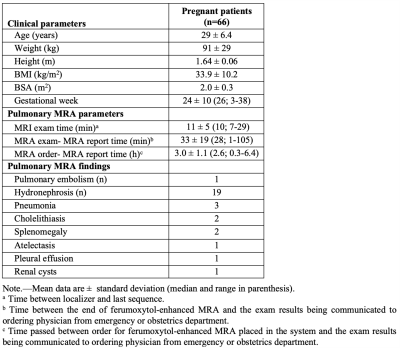
70 Fe-MRA exams were successfully performed in 66 pregnant woman (mean age 29 years; gestational age range 3-38 weeks; Table 3), on 1.5T/3T clinical MRI systems (GE Healthcare; Table 1), with four patients undergoing Fe-MRA twice during their pregnancy. All imaged patients tolerated the Ferumoxytol infusion well. Figure 1 demonstrates an Fe-MRA in a pregnant patient (no PE). The mean Fe-MRA exam time was 11min (range 7-29min; Table 3).Image quality analysis showed a mean score of 3.2±0.8. 58/70 exams (83%) were excellent or good and 10/70 (14%) exams were fair image quality. 2/70 (3%) exams had poor image quality (non-diagnostic), due to patient habitus and motion artifacts. The ICC of the image quality score was 0.8, showing a good reader agreement.
Overall, there was a high confidence for diagnosing PE with mean score of 3.6±0.7, 3.5±0.8 and 3.3±1.0, for three readers respectively. In 32/70 (46%) Fe-MRA exams, all readers were absolutely confident (score 4) about the diagnosis. In 4/70 (6%) exams, the lowest confidence score was given by at least one of the readers.
PE was diagnosed in 1/66 (1.5%) patients by all readers (Figure 2). Two patients had equivocal findings reported by two readers, but a follow-up CTA excluded PE in both cases. 29/66 patients had incidental findings such as pneumonia (Table 3).
During ferumoxytol infusion, one patient experienced mild symptoms including diaphoresis, nausea, flushing and coughing. The infusion was discontinued, 50mg of diphenhydramine IV was administered, and patient recovered quickly without further incident. As the ferumoxytol was not fully administered, the patient did not undergo MRI. Notably, the patient reported had no complications during an Fe-MRA performed two years earlier.
Discussion
In this study, we describe our clinical experience with Fe-MRA as an ionizing radiation-free, gadolinium-free alternative for diagnosing PE in pregnancy. Ferumoxytol use for MRA is off-label, although increasingly accepted for clinical MR application. We also note that our obstetricians consider Ferumoxytol as an option to treat iron-deficiency anemia in pregnancy, and its usage as a diagnostic imaging agent is a straightforward extension. Further studies with larger cohorts are needed to evaluate the diagnostic accuracy and Fe-MRA safety in pregnancy.Conclusion
Ferumoxytol-enhanced MRA is a feasible alternative for diagnosis of pulmonary embolism during pregnancy.Acknowledgements
The authors wish to acknowledge support from GE Healthcare and Bracco Diagnostic who provide research support to the University of Wisconsin. Dr. Reeder is a Romnes Faculty Fellow, and has received an award granted by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Pahade JK, Litmanovich D, Pedrosa I, Romero J, Bankier AA, Boiselle PM. Imaging Pregnant Patients with Suspected Pulmonary Embolism: What the Radiologist Needs to Know. RadioGraphics. 2009;29(3):639-654. doi:10.1148/rg.293085226
2. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. doi:10.1183/13993003.01647-2019
3. Benson DG, Schiebler ML, Repplinger MD, et al. Contrast-enhanced pulmonary MRA for the primary diagnosis of pulmonary embolism: current state of the art and future directions. BJR. 2017;90(1074):20160901. doi:10.1259/bjr.20160901
4. Schiebler ML, Nagle SK, François CJ, et al. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: Clinical outcomes at 3 months and 1 year: Effectiveness of MRA for PE Primary Diagnosis. J Magn Reson Imaging. 2013;38(4):914-925. doi:10.1002/jmri.24057
5. Nagle SK, Schiebler ML, Repplinger MD, et al. Contrast enhanced pulmonary magnetic resonance angiography for pulmonary embolism: Building a successful program. European Journal of Radiology. 2016;85(3):553-563. doi:10.1016/j.ejrad.2015.12.018
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA. 2016;316(9):952. doi:10.1001/jama.2016.12126
7. U.S.Food & Drug Administration. Feraheme (ferumoxytol) Information. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/feraheme-ferumoxytol-information
8. Knobloch G, Colgan T, Wiens CN, et al. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T: Investigative Radiology. 2018;53(5):257-263. doi:10.1097/RLI.0000000000000434
9. Knobloch G, Colgan T, Schiebler ML, et al. Comparison of gadolinium‐enhanced and ferumoxytol‐enhanced conventional and UTE‐MRA for the depiction of the pulmonary vasculature. Magn Reson Med. 2019;82(5):1660-1670. doi:10.1002/mrm.27853
10. Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005;21(1):46-52. doi:10.1002/jmri.20235
11. Hope MD, Hope TA, Zhu C, et al. Vascular Imaging With Ferumoxytol as a Contrast Agent. American Journal of Roentgenology. 2015;205(3):W366-W373. doi:10.2214/AJR.15.14534
12. Nguyen K-L, Yoshida T, Kathuria-Prakash N, et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology. 2019;293(3):554-564. doi:10.1148/radiol.2019190477
13. FDA Drug Safety Communication. FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warnings-and-changes-prescribing-instructions-decrease
14. Vasanawala SS, Nguyen K-L, Hope MD, et al. Safety and Technique of Ferumoxytol Administration for MRI. Magn Reson Med. 2016;75(5):2107-2111. doi:10.1002/mrm.26151
Figures