0157
Abnormal cerebrovascular reactivity in human immunodeficiency virus-infected patients with or without smoking: a resting-state fMRI study1Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Human immunodeficiency virus (HIV) infection is associated with neurodegeneration, but its effect on cerebrovascular function is not well understood. In a cohort of 160 participants, we investigated the effects of HIV and smoking on cerebrovascular reactivity (CVR) measured with standard resting-state blood oxygenation level dependent (BOLD)-fMRI. Across four participant groups (HIV+/HIV- x smokers/nonsmokers), both HIV-infection and smoking status altered CVR, but their effects were different across brain regions. Furthermore, lower nadir CD4 predicted lower thalamus CVR.
Introduction
Human immunodeficiency virus (HIV) infection can be associated with neurocognitive degeneration and decline.1 However, little is known about its effect on neurovascular function. A few recent studies suggested dampened cerebrovascular reactivity (CVR) in HIV-infected individuals.2,3 The typical CVR mapping techniques require CO2 inhalation2 or acetazolamide administration;3 hence, these studies had limited sample size and did not account for potential risk factors such as smoking. Smoking has a higher prevalence (40-60%) in persons with HIV-infection (PWH) than in the general population and is an independent risk factor for cerebrovascular disease.4 Therefore, we aimed to investigate the neurovascular function in PWH with or without tobacco smoking, using a newly developed CVR mapping technique based on resting-state blood-oxygenation level dependent (BOLD)-fMRI data. This technique exploits resting-state BOLD fluctuations due to natural fluctuations in breathing pattern.5,6 In this study, we compared CVR in major brain regions among four groups (HIV+/HIV- x smokers/nonsmokers) and explored the correlations between CVR with HIV disease severity (viral load and CD4 cell count) or smoking duration.Methods
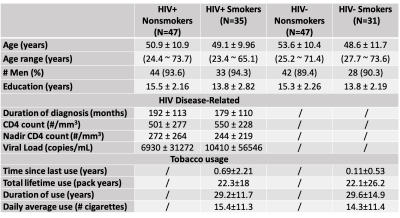
Data collected from a cross-sectional HIV study of 160 participants were retrospectively analyzed. These well-characterized participants were divided into four groups: HIV+ nonsmokers, HIV+ smokers, HIV- nonsmokers and HIV- smokers. Demographic and clinical information of the participants is shown in Table 1. In each participant, standard resting-state BOLD fMRI (TR/TE=3000/30ms, resolution=3x3x3m3) and structural T1-MPRAGE (TR/TE=2000/4.48ms, resolution=1x1x1mm3) scans were acquired on a 3T Siemens Scanner.CVR imaging analysis using the resting-state fMRI data followed the method established previously.5,6 Briefly, after motion correction and spatial smoothing (8mm FWHM kernel), BOLD images were temporally filtered to [0,0.1164Hz].6 Using the whole-brain-averaged time course as the independent variable, voxel-wise regression analysis was employed to calculate a CVR index map in % signal change, which was then normalized to the whole-brain average to yield a relative CVR map. Nine brain regions of interest (ROIs), namely the frontal lobe, parietal lobe, temporal lobe, limbic lobe, occipital lobe, insula, basal ganglia, thalamus, and basal forebrain, were segmented by an automated atlas on the T1-MPRAGE images and applied to the relative CVR map to calculate mean regional values.
Statistical analysis: A two-way analysis of variance was conducted to compare the main effects of HIV status and smoking status and the interaction between HIV and smoking status on rs-CVR data in each ROI. A subsequent post hoc analysis using Tukey’s HSD test was performed in those ROIs that showed significant main or interaction effects. As an exploratory step, in ROIs where significant ANOVA results were found, regression analyses were also performed in the HIV-infected participants to assess the association between CVR and nadir CD4 count, and in the smokers to assess the association between CVR and lifetime smoking duration.
Results
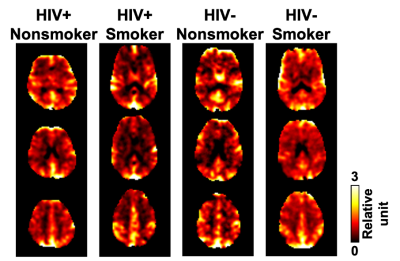
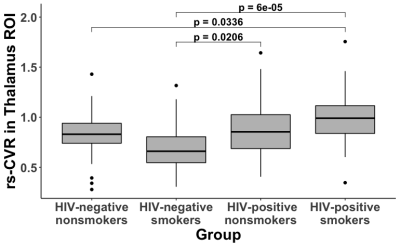
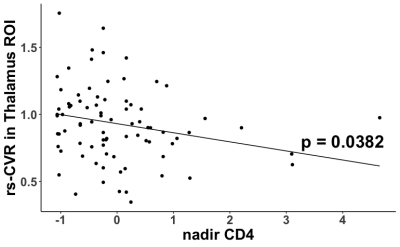
Figure 1 shows examples of relative CVR maps obtained from representative participants from the four groups. Four of the nine ROIs showed significant main effects in the ANOVA model. Specifically, the thalamus showed a significant main effect of HIV status (p = 0.0005), with higher CVR in HIV+ subjects (0.927±0.279) than HIV- subjects (0.783±0.243), and a significant interaction effect between HIV status and smoking status (p = 0.0033). Tukey’s HSD uncovered three significant post hoc comparisons (Figure 2). The frontal lobe showed a significant main effect of smoking status (p=0.0143), with higher CVR in smokers (0.991±0.103) than in nonsmokers (0.944±0.130). Tukey’s HSD uncovered significant differences between HIV- smokers and HIV- nonsmokers (p=0.0300), and between HIV- smokers and HIV+ nonsmokers (p=0.0457). The limbic lobe showed a significant main effect of HIV status (p=0.0094) and a significant main effect of smoking status (p=0.0401), and Tukey’s HSD showed a significant difference between HIV+ smokers and HIV- nonsmokers (p=0.0033). The basal forebrain showed a significant main effect of HIV status (p=0.0027) and Tukey’s HSD showed a significant difference between HIV- smokers and HIV+ smokers (p=0.0236).Among the HIV-infected participants, exploratory regression analysis revealed that lower nadir CD4 predicted higher CVR in the thalamus (β=-0.0678, p=0.0382, Figure 3). Among the smokers, a significant interaction between age and smoking duration (β=0.0621, p=0.011) was found on the CVR in the basal forebrain.
Discussion
In this study, CVR maps were extracted from standard resting-state BOLD-fMRI scans. Although this approach does not allow the quantification of absolute CVR values (in the units of %ΔBOLD/mmHg CO2), it enables the assessment of regional variations in CVR changes. Our results suggest that HIV-infection and smoking alter vascular function spatially selectively. Among the regions examined, smoking has a larger effect on frontal lobe CVR, whereas HIV has larger effects on thalamus, limbic and basal forebrain CVR. In the thalamus, CVR was further found to be associated with nadir CD4 count which is a reliable predictor of neurocognitive changes associated with HIV infection,7,8 suggesting that alteration of vascular function in the thalamus may contribute to neurodegeneration in HIV-infection. Further studies will be performed to test this hypothesis.Conclusion
Resting-state CVR mapping provides an effective tool to investigate neurovascular function alterations in HIV-infection. Both HIV-infection and smoking alter CVR, but their effects are different across brain regions.Acknowledgements
No acknowledgement found.References
1. Chang L, Jiang C, Cunningham E, et al. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014 Jun 17;82(24):2213-22.
2. Chow F, Boscardin WJ, Mills C, et al. Cerebral vasoreactivity is impaired in treated, virally suppressed HIV-infected individuals. AIDS. 2016 Jan 2;30(1):45-55.
3. Callen A, Dupont S, Pyne J, et al. The regional pattern of abnormal cerebrovascular reactivity in HIV-infected, virally suppressed women. J Neurovirol. 2020 Oct;26(5):734-742.
4. Rahmanian S, Wewers ME, Koletar S, et al. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc. 2011 Jun;8(3):313-9.
5. Liu P, Li Y, Pinho M, et al. Cerebrovascular reactivity mapping without gas challenges. Neuroimage. 2017 Feb 1;146:320-326.
6. Liu G, Lu H, Li Y, et al. Cerebrovascular reactivity mapping using resting-state fMRI: comparison with CO2-inhalation method in 170 controls and 50 Moyamoya patients. Proceedings of ISMRM 2020 Annual Meeting. 2020;p.1068
7. Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751.
8. Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol. 2006 Oct;12(5):387–91.
Figures