0155
Functional network degeneration is associated with blood neurofilament light and cognitive decline in autosomal dominant Alzheimer disease1Washington University in St. Louis, St. Louis, MO, United States, 2St. Louis University, St. Louis, MO, United States, 3University of Tubingen, Tubingen, Germany
Synopsis
Research suggests that serum neurofilament light (NfL), an indirect measure of neuronal cell death, is associated with volumetric and white matter changes, and is predictive of cognitive decline in Alzheimer disease (AD). We report that NfL is associated with default mode network (DMN) functional connectivity as well as DMN connectivity with control networks. DMN connectivity with control networks is additionally associated with concurrent cognition. Hierarchical regression demonstrates NfL, DMN, and Aβ-amyloid each contribute to predicting cognition. These findings suggest NfL is an indirect marker of functional network degeneration and both NfL and DMN connectivity are distinct biomarkers of AD progression.
Introduction
Alzheimer disease (AD) is characterized by progressive memory loss and cognitive decline. Recent research suggests that neurofilament light (NfL), an indirect measure of neuronal cell death, is measurable in the blood, and is predictive of cognitive decline in individuals with autosomal dominant AD (ADAD).1 While research suggests that blood estimates of NfL correspond to volumetric and white matter changes in the brain, less is known about the correspondence between NfL and functional connectivity.2AD has been characterized as a disconnection syndrome by which progressive loss of connectivity between brain regions produces cognitive decline and onset of dementia.3 The default mode network (DMN) functional connectivity (FC) is most often implicated in AD because of its spatial colocalization with beta amyloid deposition.4-9 As the disease progresses, however, other functional networks outside of the DMN degenerate including the salience and executive-control networks.10-13
The Dominantly Inherited Alzheimer Network at Washington University has collected data on 380 people from families with mutations that cause an overproduction of Aβ-amyloid which irrevocably leads to dementia at a young age. Crucially, the consistent age of symptom onset within families and mutation types allows participants to be staged relative to their expected dementia onset. Leveraging this unique feature of ADAD, we aimed to assess how NfL levels are associated with increasing FC disruption within and between functional brain networks. We hypothesized that FC network disruption would predict cognitive function.
Methods
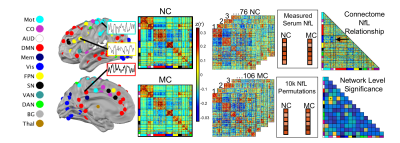
We performed neuroimaging, cognitive, and blood serum NfL assessments in a longitudinal sample of 76 mutation non-carriers (NC) and 106 mutation carriers (MC) age 20-65, with a range of estimated years from onset (EYO) of symptoms (EYO mean = -5.5). Serum blood samples were collected, centrifuged, frozen, and shipped to the University of Tübingen where they were assayed in duplicate for NfL. The Mini-Mental State Exam (MMSE) was used as a measure of cognitive decline.14 Pittsburgh Compound B administered during PET imaging was used to assess Aβ-amyloid. Aβ data were converted to regional standardized uptake value ratios relative to the cerebellar grey matter using FreeSurfer-derived ROIs.15,16 A global measure of mean cortical uptake of Aβ burden was derived from cortical regions that have been shown to have elevated signal in Alzheimer’s Disorder.15Resting state functional MRI (rs-fMRI) data were acquired on 3T Siemens TIM Trio or Verio scanners (TR/TE=2200/30ms; voxels 3.125×3.125×3.3mm3). T1-weighted images (TR/TE=2300ms; voxels 1.1 X 1.1 X 1.2 mm3) were acquired for registration. For each subject, five minutes of low-motion rs-fMRI data were included (scrubbing with framewise displacement <0.2 mm).17 FC was estimated using zero-lag Pearson correlations calculated between 246 regions of interest throughout the cortex and subcortical areas, and organized into canonical FC networks (Fig 1).18,19
We examined associations between FC and NfL using a Network Level Analysis (NLA) approach.18,20 Briefly, NLA used non-parametric correlations to determine associations between FC and MMSE scores for MC and NC adults (Figure 1). Next, correlations were thresholded and binarized for each group within and between network pairs, and network level associations with NfL were determined using Chi-squared and hypergeometric tests. Network significance was determined via randomization (p<.05).
Results
As expected, MC had greater serum NfL measurements than NC (p<0.05) (Figure 2A). While MC and NC did not differ in age, serum NfL was positively correlated with age in both groups (p<0.001) (Figure 2D&E). Thus, variance due to age was regressed out of serum NfL and FC data prior to analysis. In the MC group, NfL was positively correlated with Aβ-amyloid and negatively correlated with MMSE scores. Given the correlation between NfL and age, partial correlations for all FC network analyses were conducted controlling for age.Edgewise FC between each pair of ROI in each network was correlated with NfL in both MC and NC groups after regressing out effects due to age (Figure 3A). Connectivity among six networks were significantly correlated with NfL in MC. These network level associations included within-network connectivity of DMN and auditory network as well as between-network connectivity of visual-memory, DMN-cingulo-opercular (CO), DMN-salience network (SN), and DMN-dorsal attention (DAN). Four of these networks showed stronger coupling between FC and NfL in MC compared to NC including DMN within network connectivity, and DMN connectivity with CO, SN, and DAN (Figure 3B&C).
These same four network pairs were also correlated with MMSE scores (Figure 4). Specifically, greater DMN within network was positively correlated with MMSE while DMN between network FC with executive-control networks was negatively correlated with MMSE scores. Further, hierarchical linear regression was conducted in the MC group. This model demonstrated significant contributions from NfL, DMN FC, and Aβ-amyloid in explaining individual variability in concurrent MMSE scores when accounting for age and education (Table 1).
Discussion and Conclusions
NfL was associated with reduced FC within the DMN and between DMN and executive-control networks. Further, DMN within network and DMN between network FC with control networks was associated with cognitive decline. NfL, DMN FC, and Aβ-amyloid each independently contributed to variance in cognitive function. The present study suggests that reduced FC in Alzheimer’s disease underlies progression of cognitive decline and blood serum NfL is an indirect biomarker of reduced DMN within network and DMN between network FC.Acknowledgements
Study funding support was provided by NIH grants: K99 EB029343, TL1 TR002344, T32 MH100019, K01 MH103594, K01 AG053474, UF1 AG032438, P30 NS098577, and the German Center for Neurodegenerative Diseases (DZNE).References
1 Preische, O. et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nature Medicine 25, 277-283, doi:10.1038/s41591-018-0304-3 (2019).
2 Millar, P. R. et al. Evaluating resting-state BOLD variability in relation to biomarkers of preclinical Alzheimer's disease. Neurobiology of Aging 96, 233-245, doi:10.1016/j.neurobiolaging.2020.08.007 (2020).
3 Brier, M. R., Thomas, J. B. & Ances, B. M. Network dysfunction in Alzheimer's disease: refining the disconnection hypothesis. Brain Connect 4, 299-311, doi:10.1089/brain.2014.0236 (2014).
4 Bero, A. W. et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14, 750-756, doi:10.1038/nn.2801 (2011).
5 Buckner, R. L. et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709-7717, doi:10.1523/JNEUROSCI.2177-05.2005 (2005).
6 Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1-38, doi:10.1196/annals.1440.011 (2008).
7 Drzezga, A. et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134, 1635-1646, doi:10.1093/brain/awr066 (2011).
8 Mattsson, N., Cullen, N. C., Andreasson, U., Zetterberg, H. & Blennow, K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 76, 791-799, doi:10.1001/jamaneurol.2019.0765 (2019).
9 Mormino, E. C. et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex 21, 2399-2407, doi:10.1093/cercor/bhr025 (2011).
10 Dai, Z. et al. Disrupted structural and functional brain networks in Alzheimer's disease. Neurobiol Aging 75, 71-82, doi:10.1016/j.neurobiolaging.2018.11.005 (2019).
11 Agosta, F. et al. Resting state fMRI in Alzheimer's diesease: beyond the default mode network. Neurobiology of Aging 33, 1564-1578 (2011).
12 Brier, M. R. et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. Journal of Neuroscience 32, 8890-8899, doi:10.1523/JNEUROSCI.5698-11.2012 (2012).
13 Thomas, J. B. et al. Functional connectivity in autosomal dominant and late-onset Alzheimer disease. JAMA Neurology 71, 1111-1122, doi:10.1001/jamaneurol.2014.1654 (2014).
14 Folstein, M. F., Folstein, S. E. & McHugh, P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12, 189-198, doi:10.1016/0022-3956(75)90026-6 (1975).
15 Su, Y. et al. Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One 8, e73377, doi:10.1371/journal.pone.0073377 (2013).
16 Su, Y. et al. Partial volume correction in quantitative amyloid imaging. Neuroimage 107, 55-64, doi:10.1016/j.neuroimage.2014.11.058 (2015).
17 Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320-341, doi:10.1016/j.neuroimage.2013.08.048 (2014).
18 Wheelock, M. D. et al. Altered functional network connectivity relates to motor development in children born very preterm. NeuroImage 183, 574-583 (2018).
19 Seitzman, B. A. et al. A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. Neuroimage, 116290, doi:10.1016/j.neuroimage.2019.116290 (2019).
20 Eggebrecht, A. T. et al. Joint Attention and Brain Functional Connectivity in Infants and Toddlers. Cereb Cortex 27, 1709-1720, doi:10.1093/cercor/bhw403 (2017).
Figures