0154
Utility of Adiabatic T1Ρ and T2Ρ Mapping to Detect Ischemic Injury to the Femoral Head: An In Vivo Piglet Model Study at 3T MRI1Department of Veterinary Clinical Sciences, University of Minnesota, Saint Paul, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Adiabatic T1ρ and T2ρ are potentially advantageous relaxation time mapping techniques for in vivo imaging of the musculoskeletal system. In this work, we compared adiabatic T1ρ and T2ρ mapping to T2 and continuous-wave T1ρ mapping in a piglet model of osteonecrosis of the femoral head. We found that adiabatic T2ρ had a robust increase in response to ischemic injury to the bone marrow, bone, and epiphyseal cartilage of the ischemic femoral head compared to the contralateral healthy femoral head. Adiabatic T2ρ may be a useful technique to detect early-stage injury in ischemic bone and joint disorders.
Introduction
Quantitative mapping of T2 and continuous-wave T1ρ (cwT1ρ) relaxation times has recently been shown, in ex vivo and in vivo piglet model studies, to be sensitive in detecting ischemic injury to the femoral head [1-3]. Specifically, T2 and cwT1ρ were significantly increased in the secondary ossification center (SOC; i.e., bone marrow and trabecular bone) and the overlying vascularized epiphyseal cartilage of ischemic vs. contralateral-control femoral heads as early as 48 hours after surgical induction of ischemia. These non-contrast-enhanced techniques may be clinically useful in detecting and characterizing early-stage ischemic injury, improving our ability to diagnose, stage, and inform treatment of Legg-Calvé-Perthes disease [4], osteonecrosis of the femoral head [5], and other ischemic bone and joint disorders. Adiabatic T1ρ (αT1ρ) and adiabatic T2ρ (αT2ρ) are alternative, quantitative mapping methods that may have advantages compared to T2 and cwT1ρ for in vivo imaging at 3T MRI [6,7]. These techniques have been shown to be sensitive to articular cartilage degeneration and may provide complementary information to T2 and cwT1ρ [8,9]. Furthermore, compared to cwT1ρ, αT1ρ and αT2ρ can be less demanding on clinical scanner hardware, reduce RF heating, and enable a broader range of spin-lock frequencies to be probed [6,7]. The purpose of the present study was to compare the sensitivity of T2, cwT1ρ, αT1ρ, and αT2ρ in detecting ischemic injury to the SOC and epiphyseal cartilage of the femoral head using an in vivo piglet model at clinical 3T MRI.Methods
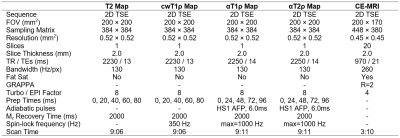
Animal Model: This study was approved by our institution’s IACUC. Six-week-old piglets (n=5) underwent unilateral surgery to completely interrupt the vascular supply to one of the femoral heads by encircling the femoral neck with a tight ligature and transecting the ligamentum teres [10]. The contralateral femoral head was unaltered and served as a normal control.In Vivo 3T MRI: One week after surgery, the piglets were sedated and anesthetized for in vivo imaging at 3T MRI (Siemens Prisma) using the body coil for transmit and small flex coils for receive. A magnetization-prepared 2D TSE sequence was used to image the bilateral femoral heads with T2, cwT1ρ, αT1ρ, and αT2ρ mapping. To confirm surgical induction of femoral head ischemia, subtraction contrast-enhanced MRI was subsequently acquired using a T1-weighted TSE sequence and intravenous administration of gadolinium contrast material (0.2 mmol/kg gadoteridol). Scan parameters are shown in Table 1.
Histology: Two of the five piglets were euthanized immediately after imaging, and the femoral heads were collected for histological analysis. Each femoral head was bisected, fixed in 10% NBF, decalcified in 10% EDTA, cut into a 3.0 mm slab, and a 5.0 µm thick section was stained with H&E.
Data Analysis: Quantitative relaxation time maps were computed in Matlab by fitting a mono-exponential signal decay model. Median relaxation times were then calculated in two regions of interest (ROIs): (i) the SOC, which consists of the bone marrow and trabecular bone of the femoral head; and (ii) the overlying epiphyseal cartilage. Median ROI values were compared between the operated and control femoral heads using paired t-tests with an exploratory significance threshold of p<0.05. Furthermore, the paired differences between the ischemic and control femoral heads were compared among different relaxation times using the Pearson correlation coefficient r.
Results
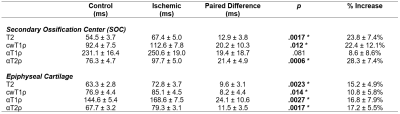
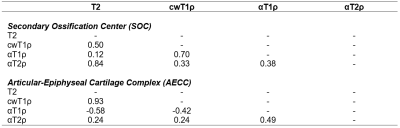
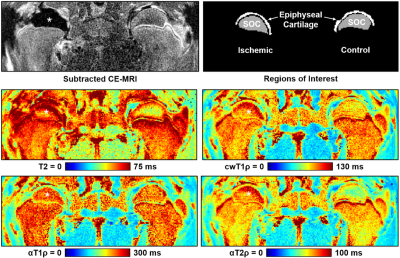
Contrast-enhanced MRI confirmed a lack of femoral head perfusion in all five piglets. Histological analysis of the ischemic femoral heads identified diffuse necrosis of bone marrow cells and necrosis of some osteocytes in the SOC and necrosis of chondrocytes in the calcified, hypertrophic, and a portion of the proliferative zones of the epiphyseal cartilage. Quantitative ROI results are shown in Tables 2 and 3. T2, cwT1ρ, and αT2ρ were all increased in the SOC of the operated vs. control femoral heads, while αT1ρ was notably less sensitive to the ischemic injury. While T2 and αT2ρ (r=0.84) and cwT1ρ and αT1ρ (r=0.70) were strongly correlated in the SOC, the other pairs of relaxation times were not. In the AECC, all four relaxation times were increased in the operated vs. control femoral heads, with T2 and αT2ρ having the most robust response. While T2 and cwT1ρ were strongly correlated (r=0.93), the other pairs (notably including T2 and αT2ρ) were not. Quantitative maps for one of the piglets are shown in Figure 1, demonstrating a clear increase in relaxation times in the ischemic vs. control femoral heads.Discussion
Our results support that αT2ρ is sensitive in detecting ischemic injury to the SOC and the overlying vascularized epiphyseal cartilage. αT1ρ was also sensitive in detecting ischemic injury to the epiphyseal cartilage. Interestingly, of all four relaxation times studied, αT2ρ had the greatest overall response to ischemic injury in both the SOC and epiphyseal cartilage. The lack of strong correlations between many of the relaxation times suggests that they may be sensitive to different components of the ischemic injury related to their different contrast mechanisms; thus, the techniques may provide complementary information to each other and, in combination, a more specific assessment of the severity of tissue injury. In conclusion, αT2ρ may be a useful complementary relaxation time mapping technique to T2 and cwT1ρ to detect early-stage ischemic injury to bone marrow, bone, and epiphyseal cartilage.Acknowledgements
We thank Dee Koski, Kathy Stuebner, Amber Winter, Kelly Bergsrud, Andrea Chehadeh, and Sara Pracht for their assistance with the animal studies. This study was supported by the National Institutes of Health (K01AR070894, UL1TR002494, K01OD021293, and P41EB027061). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
1. Johnson CP, Wang L, Tóth F, Aruwajoye O, Carlson CS, Kim HK, Ellermann JM. Quantitative MRI helps to detect hip ischemia: preclinical model of Legg-Calvé-Perthes disease. Radiology 2018; 289(2):386-395.
2. Johnson CP, Toth F, Armstrong AR, Kim HK, Ellermann JM. Quantitative T2, T1ρ, and diffusion mapping of early-stage ischemic osteonecrosis of the femoral head: an in vivo piglet model study at 3T MRI. Proc 28th ISMRM 2020; No. 1145.
3. Johnson CP, Bhave S, Armstrong AR, Chase KL, Carlson CS, Ellermann JM, Kim HK, Tóth F. Quantitative T2 and T1ρ mapping detect early ischemic injury to the epiphyseal cartilage of the femoral head at 3T MRI: an in vivo piglet model study. Proc ORS Annual Meeting 2021 (accepted).
4. Kim HK. Pathophysiology and new strategies for the treatment of Legg-Calve-Perthes disease. J Bone Joint Surg Am 2020; 94:659-669.
5. Larson E, Jones LC, Goodman SB, Koo KH, Cui Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop 2018; 42(7):1723-1728.
6. Mangia S, Liimatainen T, Garwood M, Michaeli S. Rotating frame relaxation during adiabatic pulses vs. conventional spin lock: simulations and experimental results at 4 T. Magn Reson Imaging 2009; 27:1074-1087.
7. Gilani IA, Sepponen R. Quantitative rotating frame relaxometry methods in MRI. NMR Biomed 2016; 29(6):841-61.
8. Rautiainen J, Nissi MJ, Salo EN, Tiitu V, Finnilä MAJ, Aho OM, Saarakkala S, Lehenkari P, Ellermann J, Nieminen MT. Multiparametric MRI assessment of human articular cartilage degeneration: Correlation with quantitative histology and mechanical properties. Magn Reson Med 2015; 74:249-259.
9. Casula V, Nissi MJ, Podlipská J, Haapea M, Koski JM, Saarakkala S, Guermazi A, Lammentausta E, Nieminen MT. Elevated adiabatic T1ρ and T2ρ in articular cartilage are associated with cartilage and bone lesions in early osteoarthritis: A preliminary study. J Magn Reson Imaging 2017; 46:678-689.
10. Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84-A(8):1329-34.
Figures