0142
Ultrafast DCE MRI for post-NST evaluation of breast cancer1Graduate School of Medicine, Kyoto University, Kyoto, Japan, 2Institute for Advancement of Clinical and Translational Science (iACT), Kyoto University Hospital, Kyoto, Japan, 3Department of Radiology, National Hospital Organization Kyoto Medical Center, Kyoto, Japan, 4MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 5Department of Diagnostic Pathology, Kyoto University Hospital, Kyoto, Japan, 6Department of breast surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Synopsis
The study evaluated the accuracy for predicting pathologic complete response (pCR) after neo-adjuvant systemic therapy (NST) using ultrafast dynamic contrast-enhanced (UF-DCE) MRI. The receiver operating characteristics (ROC) analysis for the presence of residual lesion revealed higher diagnostic performance of UF-DCE MRI compared with conventional dynamic contrast-enhanced (DCE) MRI overall and in the group of triple negative subtype. The deviation from pathology was smaller for UF-DCE MRI derived sizes compared to conventional DCE MRI overall and in luminal group. UF-DCE MRI potentially assesses the post-NAC status in breast cancer patients accurately in a shorter acquisition time.
Introduction
For breast cancer patients after neo-adjuvant systemic therapy (NST), pathological complete response (pCR) is known to be an important prognostic factor. Additionally, effort has been made to omit surgery for those who achieved pCR to avoid overtreatment (1,2). Dynamic contrast-enhanced (DCE) MRI is used to evaluate treatment response, but sometimes fails to predict pCR due to enhancing scar or inflammation (3–5). Ultrafast DCE (UF-DCE) MRI can be useful in accurately detecting patients with pCR (6), while diagnostic performance may vary by subtype. This study aimed to evaluate the diagnostic performance of pCR and residual tumor size on UF-DCE MRI in comparison to other conventional DCE-MRI, with consideration of subtype.Methods
Patients and MRI setting: Our study population comprised consecutive 55 female patients (49.7 years old on average) who underwent NST for invasive breast cancer and following pre-operative breast DCE MRI with the UF-DCE protocol from April 2016 to October 2020. MR examinations were performed using a 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 16-channel dedicated bilateral breast coil. Gadobutrol (Gadovist, Bayer, Germany) was intravenously infused at a dose of 0.1 ml/kg and at a rate of 2.0 ml/sec, followed by 20 ml of saline at the same rate. The DCE MR protocols were as follows: 1. pre phase; 2. UF-DCE MRI (15 seconds before-60 seconds after contrast injection, 2 seconds preparation time followed by 3.7 seconds/phase x continuous 20 phases); 3. Early phase of DCE MRI, 60-120 sec after contrast injection (EP); 4. high spatial-resolution contrast enhanced MRI, 120-300 seconds after contrast injection (HR); 5. Delayed phase of DCE MRI, 300-360 seconds after contrast injection (DP). UF-DCE MRI was acquired with a prototype based on the 3D gradient-echo volumetric interpolated breath-hold examination (VIBE) sequence using a compressed sensing (CS) reconstruction (TR/TE 5.0/2.5 ms, FA 15 degrees, FOV 360 mm×360 mm, matrix 384×269, thickness 2.5 mm, CS acceleration=16.5, temporal resolution 3.7 seconds per phase, 20 phases, iteration number=30). Images of the 20th phase of UF-DCE MRI (UF), EP, DP and HR were used for the image evaluation.Image evaluation: Two independent radiologists evaluated images of UF, EP, DP and HR in this order, and recorded the maximum length of the residual enhancing area in the axial plane. When no enhancing area was observed, it was considered to be complete response and the length was recorded as zero. They were allowed to refer to the pre-NST MRIs.
Statistical analysis: The following two analyses were performed for the entire population and for groups by subtype (luminal, human epidermal growth factor receptor 2 (HER2)-enriched and triple negative).
1. Diagnostic performance: The inter-reader agreement in the presence or absence of residual lesion was evaluated using kappa statistics. Receiver operating characteristics (ROC) analysis was performed to determine the area under the ROC curve (AUC) of each protocol for the presence of residual lesion. The reference standard was pathology, and pCR was defined as no invasive cancer, allowing in situ carcinoma.
2. Comparison in size: The inter-reader agreement for the lesion diameter was evaluated by calculating intraclass correlation coefficients (ICC). The size of the residual lesions on each protocol was compared with that on surgical specimens, and the difference between the image and pathological sizes of each protocol was compared using Wilcoxon signed-rank test with Bonferroni correction. If the pathological lesion size was under 1mm, it was calculated as 1mm. The significance level after Bonferroni correction was defined as p values < 0.05/3 = 0.0167. The average values of two readers were used for the analysis.
Results
A total of 55 lesions from 55 patients were evaluated, of which 23 lesions achieved pCR. Detailed patient characteristics were summarized in table 1.1. Diagnostic performance: The inter-reader agreement in the presence or absence of residual lesion was almost perfect for UF (kappa =0.84), moderate for EP and DP (kappa = 0.64 and 0.68) and fair for HR (kappa = 0.51). The overall AUC of UF (0.88) was the highest than the other protocols (0.71–0.85) (Figure 1). Sub-analysis by subtype showed the similar trend in triple negative group (AUC = 0.89 and 0.65–0.82 for UF and others, respectively) while not in luminal group.
2. Comparison in size: The inter-reader agreement in the lesion diameter was excellent (ICC: 0.92–0.97). The difference in image and pathological sizes on UF (5.8±8.3) was significantly smaller than EP, DP and HR overall (9.2±10.3, 10.9±10.9 and 10.5±10.6, p<0.01). Among luminal type breast cancer, the size difference was significantly smaller on UF and EP (5.8±6.1 and 9.9±10.1) than on DP and HR (11.9±11.4 and 11.0±10.8).
Representative cases are shown in figures 2–4.
Discussion
UF-DCE MRI yields better diagnostic performance for prediction of pCR with high inter-reader agreement than conventional DCE MRI. This is true for triple negative subtype while not applicable to luminal subtype. Image-based residual lesion size evaluated on UF-DCE MRI was closer to pathology than on conventional DCE MRI.The current results show the potential of UF-DCE MRI to provide more accurate assessment of post-NST status of breast cancer in a shorter acquisition time.Conclusion
UF-DCE MRI may be suitable for assessment of post-NST status of breast cancer.Acknowledgements
This work was partly supported by JSPS KAKENHI Grant Number 18K07673 "Tumor vascularity and tumor-related vessels using ultrafast DCE MRI of the Breast"References
1. Kuerer HM, Vrancken Peeters M-JTFD, Rea DW, Basik M, De Los Santos J, Heil J. Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann Surg Oncol. Springer New York LLC; 2017;24(10):2855–2862.
2. van la Parra RFD, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: Historical perspective and current trials. Breast Cancer Res. Breast Cancer Research; 2016;18(1):1–8.
3. Sener SF, Sargent RE, Lee C, et al. MRI does not predict pathologic complete response after neoadjuvant chemotherapy for breast cancer. J Surg Oncol. 2019;120(6):903–910.
4. Marinovich ML, Macaskill P, Irwig L, et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer. BMC Cancer; 2015;15(1):1–12.
5. Fukuda T, Horii R, Gomi N, et al. Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: association with breast cancer subtype. Springerplus. Springer International Publishing; 2016;5(1):1–9.
6. Honda M, Kataoka M et al, Post-NAC evaluation using ultrafast breast dynamic contrast-enhanced MRI. Proc. Int Soc Magn Reson Med 2020;0575.
Figures
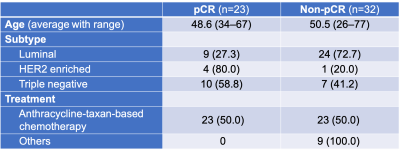
Table 1: Patient characteristics.
pCR, pathological complete response; HER2, human epidermal growth factor receptor 2. Unless otherwise specified, data in columns 2 and 3 are number of patients with percentages in parentheses.
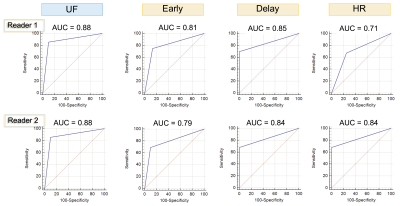
Figure 1: ROC curves of the whole population.
UF, ultrafast dynamic contrast-enhanced MRI; HR, high spatial-resolution.
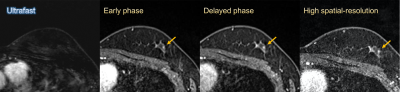
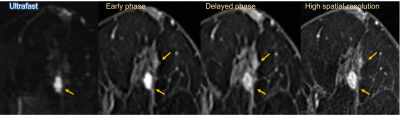
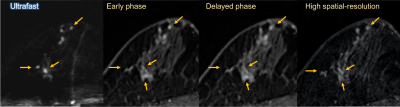
Figure 4: Post-neoadjuvant chemotherapy MRI of 51-year-old woman with luminal type breast cancer in her right breast. On surgical specimen, residual tumor consisted mainly of in-situ lesion and the size of invasive cancer was 27mm. On MRI, the lesion was evaluated larger on all images, but closer to the pathological size on ultrafast MRI (33.5mm) than early phase, delayed phase and high spatial-resolution images (41–44mm).