0141
Deregulation of lipid composition in peri-tumoural adipose tissue in postmenopausal patients with breast cancer1Institute of Medical Sciences, University of Aberdeen, Aberdeen, United Kingdom, 2Clinical Radiology, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3Radiology, Royal Marsden Hospital, London, United Kingdom, 4Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 5School of Medicine, University of Aberdeen, Aberdeen, United Kingdom, 6Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom
Synopsis
Deregulation of lipid metabolism has been shown in BRCA1/2 genetic mutation carriers. Mammary adipose tissues in postmenopausal women are the primary sites of oestrogen production linked to tumour initiation and progression. Therefore, lipid composition in postmenopausal breast plays a key role in breast cancer monitoring and subsequent development of prevention strategies. Previous studies focused on cell or animal models and invasive lipid extraction methods, while conventional MRS is inadequate in complete lipid composition measurement. We hypothesised that lipid composition in peri-tumoural breast adipose tissue is affected by the presence of tumour in postmenopausal women, using a non-invasive 2D MRS approach.
Introduction
Deregulation of lipid metabolism in breast has been shown in BRCA1/2 genetic mutation carriers1. Polyunsaturated fatty acid (PUFA) is depleted in tumour initiation, while free fatty acids (FA) are released from surrounding mammary adipocytes to support cancer growth2. Lipid composition is modulated by oestrogen linked to tumour initiation and progression3. While oestrogen is considered an endocrine hormone prior to menopause, the adipose tissue becomes the predominant source of oestrogens in postmenopausal women3. Therefore, lipid composition in postmenopausal women plays a key role in breast cancer initiation and subsequent development of prevention strategies4. Double quantum filtered correlation spectroscopy (DQF-COSY) provides accurate in vivo quantification of lipids5. We therefore hypothesised that lipid composition in the breasts of postmenopausal women is altered by the presence of adjacent tumour.Methods
Fourteen patients with invasive ductal carcinoma (age 53 – 71 years) and 15 age-matched healthy controls (age 54 – 76 years) participated in this study. Only patients undergoing wide local excision or mastectomy, with a tumour size larger than 1 cm on ultrasound were eligible. For healthy controls, women on hormone replacement therapy, having previous breast malignancy and family history of breast cancer were not eligible. The study was approved by the North of Scotland Research Ethics Committee (REC Ref: 16/NS/0077), and written informed consent was obtained from all the participants prior to the study.MRS Acquisition
All data were acquired on a 3 T whole body clinical MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using a body coil for uniform transmission and a 16-channel breast coil for high sensitivity detection. 2D MRS lipid spectra were acquired using DQF-COSY5 with TR of 552 ms, initial TE of 25 ms, a t1 increment of 1 ms, 256 increments, and voxel size of 20 × 20 × 20 mm3. In patients, a voxel (peri-tumoural) was positioned at 1 cm away from the tumour with a separate voxel (reference) in the contralateral healthy breast. In controls, a voxel was positioned in each breast. All voxels were positioned to contain primarily adipose tissue (Figure 1).
Data Processing
The spectral diagonal peaks of methyl protons at (0.9,0.9) ppm, olefinic double bond protons at (5.3,5.3) ppm, triglycerides (TRG) at (4.3,4.3) ppm, and off diagonal peaks of monounsaturated FA at (5.3,2.1) ppm and (2.1,5.3) ppm, PUFA at (5.3,2.8) ppm and (2.8,5.3) ppm were quantified in Felix software (v2007, Accelrys Inc., San Diego, USA)1. The unsaturated bond index (UBI), TRG, MUFA and PUFA were subsequently computed as the ratio between the corresponding peak volume against methyl protons. The disparities ([difference between the breasts] / [average of both breasts]) in the peak ratios were then calculated to yield d[UBI], d[TRG], d[MUFA], d[PUFA].
Histopathological Analysis
Histopathological examination was performed on excised breast tumours to determine proliferative activity marker Ki-67 and Nottingham Prognostic Index (NPI)6 in each patient.
Statistical Analysis
Statistical analysis was performed in SPSS software (Release 23.0, SPSS Inc., Chicago, IL, USA). Wilcoxon signed rank paired tests were performed between peri-tumoural region against contralateral breast in patients. Mann Whitney U tests were performed between contralateral breast in patients against the average of both breasts in controls. Independent sample t-tests were performed on disparity between patients against controls. Pearson’s correlation tests were performed between disparities in peri-tumoural lipid ratios against Ki-67 and NPI in patients. A p value < 0.05 was considered statistically significant.
Results
There were no significant differences in UBI (p = 0.826, Figure 2a), TRG (p = 0.975, Figure 2b), MUFA (p = 0.683, Figure 2c) and PUFA (p = 0.826, Figure 2d) between peri-tumoural region against contralateral breast in patients (Table 1). There were no significant differences in UBI (p = 0.793, Figure 2a), TRG (p = 0.965, Figure 2b), MUFA (p = 0.485, Figure 2c) and PUFA (p = 0.359, Figure 2d) between contralateral breast in patients against the average of both breasts in controls (Table 1).There was a significantly higher d[UBI] (t = 4.247, p = 0.0003) in patients (0.28 ± 0.11) than controls (0.13 ± 0.08) (Figure 3a, Table 1). There was a significantly higher d[TRG] (t = 2.863, p = 0.012) in patients (0.20 ± 0.15) than controls (0.08 ± 0.04) (Figure 3b, Table 1). There were no significant differences in d[MUFA] (p = 0.812, Figure 3c, Table 1) and d[PUFA] (p = 0.294, Figure 3d, Table 1) between patients and controls.
There were no significant correlations between d[UBI] against Ki-67 (p = 0.911, Figure 4a) or NPI (p = 0.554, Figure 4b). There were no significant correlations between d[TRG] against Ki-67 (p = 0.647, Figure 4c) or NPI (p = 0.236, Figure 4d).
Discussion
We found significantly higher disparities between breasts in UBI and TRG in postmenopausal patients than age-matched controls, indicating that the disparities in UBI and TRG could be two potential modifiable treatment targets critical for personalised medicine. Our work provided a comprehensive in vivo assessment of lipid deregulation in peri-tumoural adipose tissue.Conclusion
Unsaturated bond index and triglycerides in peri-tumoural breast adipose tissue are deranged as a result of the presence of tumour in postmenopausal women, offering potential treatment targets for breast cancer monitoring and prevention.Acknowledgements
The authors would like to thank Dr Matthew Clemence for clinical scientist support, Ms Angela Allan and Ms Vera Herd for nurse support, Ms Shona Davidson, Ms Linda Lett, Ms Louisa Pirie, Ms Fiona Geddes, Ms Kate Shaw, Ms Sheila Ingram for patient recruitment support, Ms Kim Blake, Ms Shona Stuart, Ms Brenda Still, Ms Dawn Younie for logistic support, and Ms Beverly MacLennan, Ms Nichola Crouch, Ms Laura Reid and Mr Mike Hendry for radiographer support. The authors would also like to thank Ms Mairi Fuller, Mr Dionysios Koufoudakis, Ms Elizabeth Smyth and Ms Beatrix Elsberger for providing access to the patients. This project was funded by Friends of Aberdeen and North Centre for Haematology, Oncology and Radiotherapy (ANCHOR). Sai Man Cheung’s PhD study was jointly supported by Elphinstone scholarship, Roland Sutton Academic Trust and John Mallard scholarship and is currently funded by Cancer Research UK. Vasiliki Mallikourti’s PhD study is supported by Tenovus Scotland PhD studentship.References
1. Ramadan S, Arm J, Silcock J, et al. Lipid and Metabolite Deregulation in the Breast Tissue of Women Carrying BRCA1 and BRCA2 Genetic Mutations. Radiology. 2015;275(3):675-682.
2. Wang YY, Attané C, Milhas D, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):1-20.
3. Brown KA. Impact of Obesity on Mammary Gland Inflammation and Local Estrogen Production. J Mammary Gland Biol Neoplasia. 2014;19(2):183-189.
4. Freed M, Storey P, Lewin AA, et al. Evaluation of Breast Lipid Composition in Patients with Benign Tissue and Cancer by Using Multiple Gradient-Echo MR Imaging. Radiology. 2016;281(1):43-53.
5. Prescot AP, Dzik- Jurasz ASK, Leach MO, Sirohi B, Powles R, Collins DJ. Localized COSY and DQF-COSY 1H-MRS sequences for investigating human tibial bone marrow in vivo and initial application to patients with acute leukemia. J Magn Reson Imaging. 2005;22(4):541-548.
6. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-410.
Figures
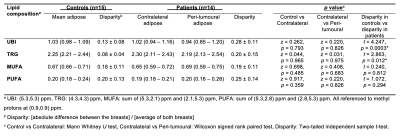
Table 1. Differences in lipid composition of breast adipose tissue between patients and controls.
Unsaturated bond index, triglycerides, monounsaturated and polyunsaturated fatty acids (UBI, TRG, MUFA and PUFA) in adipose tissue of patients and controls from double quantum filtered correlation spectroscopy (DQF-COSY). The disparity in lipid composition between patients and controls are also shown. Values are displayed as mean and standard deviation (mean ± SD) or median and interquartile range (median (IQR)). Statistically significant findings (p < 0.05) are marked by ‘*’.
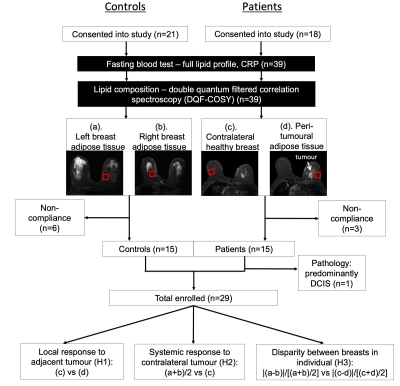
Figure 1. Study design.
A two-group cross sectional arrangement as shown in a flow chart. Twenty-one female healthy controls and 18 female breast cancer patients were eligible at initial screening and were consented into the study. All controls and patients were scanned on a clinical 3 T MRI scanner to assess the lipid composition in breasts (a, b: Controls; c, d: Patients) using double quantum filtered correlation spectroscopy (DQF-COSY). In total, 15 controls and 14 patients with invasive ductal carcinoma completed MR scans and participated in the study.
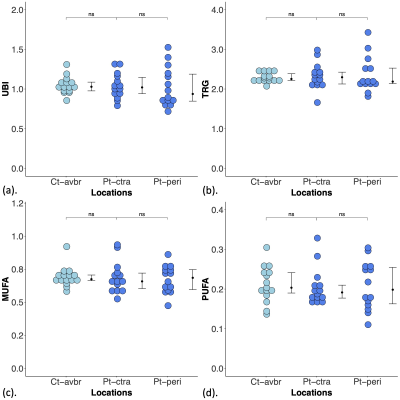
Figure 2. Lipid composition in patients and controls.
(a) Unsaturated bond index (UBI), (b) triglycerides (TRG), (c) monounsaturated fatty acids (MUFA) and (d) polyunsaturated fatty acids (PUFA) in peri-tumoural region (Pt-peri), contralateral breast (Pt-ctra) in patients and average of both breasts (Ct-avbr) in controls. The error bar indicates the median and interquartile range. Wilcoxon signed rank paired tests were performed within patients, while Mann Whitney U tests between patients and controls. There were no significant (ns) differences in lipid composition.
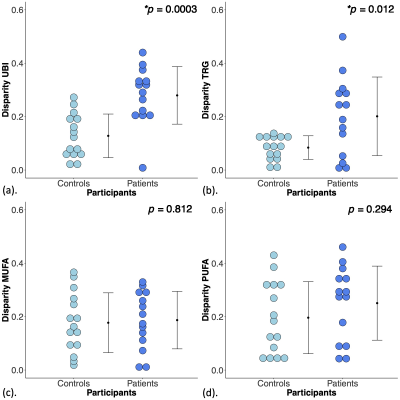
Figure 3. Disparities in unsaturated bond index, triglycerides, monounsaturated and polyunsaturated fatty acids (UBI, TRG, MUFA and PUFA) between patients and controls.
The disparity in (a) UBI, (b) TRG, (c) MUFA and (d) PUFA between patients and controls are shown in dot plots. Each dot represents the disparity between breasts in each participant, and the dots are organised in two columns corresponding to the two groups. The error bar indicates the mean and standard deviation. The independent sample t-tests were performed and p < 0.05 was considered statistically significant (‘*’).
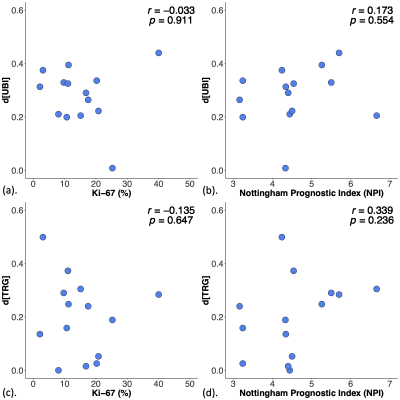
Figure 4. Association of disparities between breasts in unsaturated bond index (d[UBI]) and triglycerides (d[TRG]) against proliferative activity marker Ki-67 and Nottingham Prognostic Index (NPI) in patients.
The association between disparities in UBI (d[UBI]) and TRG (d[TRG]) against (a, c) Ki-67 and (b, d) NPI in patients. Pearson’s correlation tests were performed and corresponding r score and p value are displayed. There were no significant correlations between disparities in lipid composition against immunohistological and prognostic markers.