0139
A Hybrid DWI Approach for Simultaneous Assessment of Cellularity, Vascularity, and Heterogeneity of Breast Lesions1Center for Magnetic Resonance Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China, 4Department of Radiology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China, 5Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Breast cancer is the second most common cancer among women. Breast tissue has a variety of structural features leading to different tissue properties which are collectively linked to the pathologic state of the breast lesions. In this study we demonstrate a hybrid DWI approach for simultaneous assessment of tissue cellularity, vascularity, and heterogeneity (DISMANTLE) based on intravoxel incoherent motion (IVIM) and continuous-time random walk (CTRW) diffusion models. Our results have shown that DISMANTLE improved the differentiation among benign and malignant breast lesions compared to the classical implementation of either IVIM or CTRW model.
Introduction
Accurate characterization of breast lesions is important for efficient risk assessment and optimized treatment for breast cancer. Breast tissue has various structural features leading to different tissue properties. Among these, changes in cellularity, vascularity, and heterogeneity have been increasingly linked to the pathologic state of lesions. While DWI with apparent diffusion coefficient has become a pillar of clinical MRI for probing tissue callularity1,2, recent publications indicate that multiple tissue properties can be investigated using specific b-value ranges of the diffusion-weighted signal attenuation which exhibits both Gaussian and non-Gaussian diffusion behaviors3,4. For example, intravoxel incoherent motion (IVIM) model5 with low b-values can characterize micro-vascularity while high-b-value non-Gaussian models can probe microstructures as in the case with continuous-time random-walk (CTRW) model6,7 which can reflect micro-structural heterogeneity. Although IVIM model has been increasingly used for breast tissue characterization8, there is a paucity of high-b-value DWI studies on breast cancer. More importantly, integration of information from a full-b-value “spectrum” would likely lead to new insights beyond what each individual model can provide. In this study, we propose a hybrid DWI approach for simultaneous assessment of tissue cellularity, vascularity, and heterogeneity (DISMANTLE) based on IVIM and CTRW diffusion models to characterize malignant and benign breast lesions.Theory
The DWI signal can be characterized as the sum of perfusion- and diffusion-related signal components, FPERF(b) and FDIFF(b)9, $$ S(b)=S_{0,PERF} F_{PERF} (b)+S_{0,DIFF} F_{DIFF} (b).\tag{1}$$ By adopting this hybrid approach, we characterize the diffusion-weighted signal attenuation by approximating FPERF and FDIFF based on IVIM5,10 and CTRW7 models, respectively, as follows $$S/S_0=fe^{-bD_{perf}}+(1-f) E_α (-(bD_m )^β),\tag{2}$$ where Eα is a Mittag-Leffler function7. The approach in Eq.(2), which we call DISMANTLE, enables estimation of vascularity-related (IVIM’s perfusion fraction, f and pseudo-diffusion coefficient, Dperf), cellularity-related (CTRW’s diffusion coefficient, Dm), and heterogeneity-related (CTRW’s temporal and spatial diffusion heterogeneity, α and β) parameters with a unified formulism.Methods
Image Acquisition: We recruited 33 women with histologically confirmed breast lesions (21 malignant, 12 benign). All patients underwent axial MRI scans at 3T (GE Healthcare, Discovery MR750) with an 8-channel breast coil. DWI was performed with 11 b-values (01, 501, 1002, 3002, 5002, 8004, 11004, 15006, 20006, 25008, 30008 s/mm2; subscripts denoting NEXs; TR/TE=7000/78ms, slice thickness=5mm, FOV=32cm×32cm, and matrix=256×256). Trace-weighted images were obtained to minimize the diffusion anisotropy effect.DWI Analysis: The diffusion-weighted images were analyzed through a multi-segmented implementation of DISMANTLE with the following steps (Fig.1): Step 1) The images in the low-b-value range (0-800 s/mm2) were first analyzed with IVIM model5, $$S/S_0=fe^{(-b(D_{diff}+D_{perf} )) }+(1-f) e^{(-bD_{diff} ) }\tag{3}$$ to estimate f, Ddiff, and Dperf using a segmented fitting algorithm11 by first estimating Ddiff with a mono-exponential model at mid-range-b-values (300-800 s/mm2), followed by extrapolating the mono-exponential fit to b=0 to estimate f, and constraining Ddiff and f in Eq.(3) to obtain Dperf. Step 2) Based on the estimated f and Dperf in Step 1), the perfusion-related signal was calculated according to Eq.(2), and removed from the diffusion-weighted signal. Step 3) The remaining diffusion-related signal component at the entire-b-value range (0-3000 s/mm2) was analyzed with CTRW model7, $$S/S_0=E_α (-(bD_m )^β).\tag{4}$$ For comparison, a classical CTRW-based analysis was performed by repeating Step 3) with the use of all b-values without subtracting the perfusion-related signal component.
Statistical Analysis: The mean values of DISMANTLE-based parameters, Dperf, f, Dm, α, and β, were computed over the lesion regions of interest (ROIs). A Mann-Whitney U-test was used for the group comparison, followed by a receiver operating characteristic (ROC) analysis for differentiation among benign and malignant lesions using different combinations of the parameters with a multivariate logistic regression algorithm. For comparison, this process was repeated for two additional scenarios using:1) classical IVIM-based parameters, Dperf, Ddiff, f, and 2) classical CTRW-based parameters, Dm, α, and β.
Results
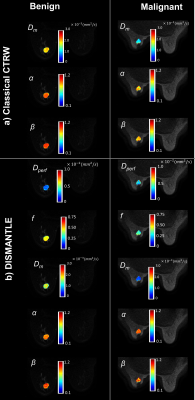
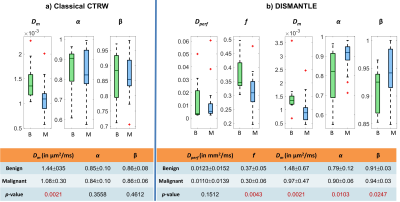
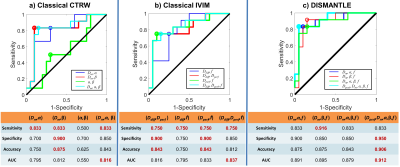
Figures 2a and 2b show parameters from the classical CTRW-based and DISMANTLE-based analysis for a representative benign (first column) and malignant (second column) lesion. Dm values were lower while α and β were higher in the DISMANTLE-based analysis compared to the classical CTRW-based analysis in both patients. The malignant lesion exhibited lower values in Dm and f; and higher values in α, β, and Dperf. While f has typically been found to be higher in malignant lesions, a significant overlap was also reported in previous studies especially when complex benign fibroadenomas with complicated cysts are involved12. In the group comparison, classical CTRW-based analysis revealed statistically significant differences (p<0.05) only in Dm, whereas all the DISMANTLE-based parameters except Dperf showed significance (Fig.3). In differentiation among the lesions, combination of DISMANTLE-based parameters produced the highest sensitivity (0.916 vs. 0.833 or 0750), specificity (0.950 vs. 0.900 or 0.900), accuracy (0.950 vs. 0.875 or 0.843), and area-under-the-curve (0.912 vs. 0.816 or 0.837) compared to the classical CTRW-based or IVIM-based parameter combinations (Fig.4).Discussion and Conclusion
We have proposed a hybrid approach that enables a comprehensive characterization of breast tissue to collectively probe tissue cellularity, vascularity, and heterogeneity from a full-b-value spectrum. Using a single representation based on a low-to-moderate-b-value IVIM and a high-b-value CTRW model, DISMANTLE improved characterization of benign and malignant breast lesions compared to the classical implementation of the models. This study emphasizes the importance of integrating information from advanced DWI for improved breast cancer diagnosis.Acknowledgements
No acknowledgement found.References
[1] Chen L, Liu M, Bao J, et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS One. 2013;8(11).
[2] Jiang R, Ma Z, Dong H, et al. Diffusion tensor imaging of breast lesions: Evaluation of apparent diffusion coefficient and fractional anisotropy and tissue cellularity. Br J Radiol. 2016;89(1064).
[3] Le Bihan D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology. 2013;268(2):318-322.
[4] Tang L and Zhou XJ. Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging. 2019;49(1):23-40.
[5] Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-407.
[6] Ingo C, Magin RL, Colon-Perez L, Triplett W, Mareci TH. On random walks and entropy in diffusion‐weighted magnetic resonance imaging studies of neural tissue. Magn Reson Med. 2014;71:617–627.
[7] Karaman MM, Sui Y, Wang H, et al. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med. 2016;76(4):1149-1157.
[8] Partridge SC, Nissan N, Rahbar H, et al. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging. 2017;45(2):337-355.
[9] Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988: 168(2):497-505.
[10] Sigmund EE, Cho GY, Kim S, et al. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med. 2011;65:1437-1447.
[11] Cho GY, Moy L, Zhang JL, et al. Comparison of fitting methods and b-value sampling strategies for intravoxel incoherent motion in breast cancer. Magn Reson Med. 2015;74(4):1077-1085.
[12] Iima M and Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: Past, present, and future. Radiology. 2016;278(1):13-32.
Figures