0133
Inductively coupled detectors for optogenetic-driven focal and multiregional fMRI signal enhancement1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, University of Tuebingen, Tuebingen, Germany, 3Department of Radiology, Michigan State University, East Lansing, MI, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States
Synopsis
To improve MR detection sensitivity of multi-modal fMRI platform, we present inductive coil which could relay locally detected MR signals to the external surface coil. Under sensory stimulation, evoked whole brain EPI-based fMRI and enhanced focal laminar-specific fMRI signals were acquired with high spatiotemporal resolution (100 μm and 100 ms) using a single experimental setup. Moreover, embedding inductive coil beneath the glue-secured optical fiber and the projection-mirrored cortex on the other hemisphere boosted multiregional fMRI sensitivity to investigate the interhemispheric connectivity with laminar-specificity. This is particularly helpful to study the optogenetic-driven brain connectivity with circuit specificity at multi-modal fMRI platform.
Introduction
Optical fiber-mediated optogenetic activation in combination with functional magnetic resonance imaging (fMRI) provides a multi-modal platform to decipher brain dynamics from micro to mesoscales 1-3. However, to secure optical fiber insertion into the brain, a conventional surface coil needs to be placed above the rat brain with a certain distance separation, leading to limited MR detection sensitivity and hindering the application of multi-modal fMRI platform to specify brain physiology and pathology 4. To improve MR detection sensitivity, inductively coupled detectors 5, 6 can be placed near the region of interest to relay locally detected MR signals to the external surface coil. Here, we demonstrate an inductive coil embedded beneath the surface coil to obtain laminar-specific information for high spatiotemporal resolution (100 μm and 100 ms) in both focal rat cortex and multiregional mapping of the brain functional connectivity with the optogenetic tool. This study will make it possible to decipher brain dynamics at multi-scale with a single experimental setup, paving way for future utilization for sensitivity-enhanced multi-modal imaging.Methods
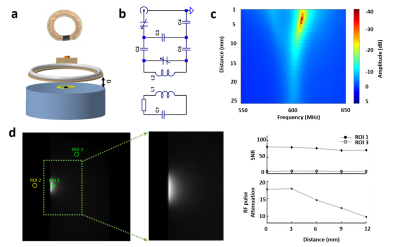
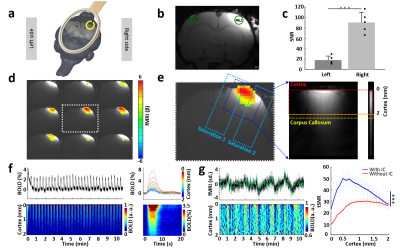
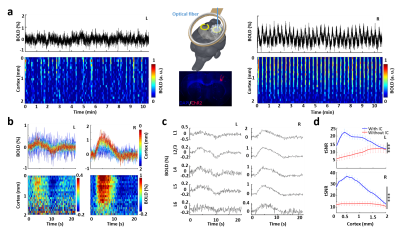
A 6-mm single loop inductive coil was developed (Fig.1a) and embedded beneath the 22-mm surface coil (Fig.1a,b) for experiments in Fig.2. For optogenetic experiments in Fig.3, one 6-mm inductive coil was placed on the right FP-S1 glued with optical fiber (200 μm), while the other one was placed on the projected FP-S1 in the left hemisphere. The FLASH based bilateral line-scanning method was combining 2 saturation RF pulses to dampen the MR signal outside the regions of interest (Fig.2e) in a 14.1-T scanner with the following parameters: TR/TE 100/9 ms, Excitation pulse angle 30°, slice thickness 1 mm, FOV 6.4 x 6.4 mm2 and matrix 64x 32. The phase-encoding gradient was turned off. Functional activation was detected by performing electrical stimulation on the left forepaw (3 Hz, 4 s, 2.5 mA) and optogenetic stimulation on right FP-S1 (2 Hz, 6 s, light pulse width 10 ms, 30 mW) to activate the neurons expressing AAV.CaMKII.ChR2.mCherry (Fig.3a). BLOCK design was 1-second pre-stimulation, 4 (6) second stimulation and 15 (13) second post-stimulation and 32 epochs for the whole trial (10 m 40 s). All line-scanning method related data analysis was performed using Matlab (Mathworks, Natick), while EPI-based fMRI data analysis was performed using AFNI (NIH, Bethesda).Results
First, a single loop inductive coil was placed on top of a 1% agarose gel phantom while the surface coil above the phantom with certain distance would provide RF excitation pulse and receive amplified signals from inductive coil (Fig.1a-c). As Fig.1d shows, the coupling effect and high focal signal enhancement from the inductive coil could be maintained for a distance as far as 12 mm with increased RF excitation power.Next, the inductive coil was evaluated in vivo for enhanced fMRI signal in the right FP-S1 region upon electrical forepaw stimulation (Fig.2a-c). As Fig.2d shows, the anatomical image with superimposed whole brain EPI-based fMRI map demonstrates the signal enhancement region that was well matched to the most activated region. Besides the EPI-based BOLD, laminar-specific BOLD was acquired with the line scanning method (Fig.2e). The time courses and BOLD map along cortical depth induced by forepaw stimulation (Fig.2f) were comparable to our previous results. Moreover, the tSNR for resting-state brain fluctuation illustrates the inductive coil is up to 2-fold more sensitive than our previous results detected with the surface coil (Fig.2g).
Last, the inductive coil was combined with optogenetic tool to demonstrate its impressive performance on multi-modal fMRI platform. Upon light stimulation, as expected, robust activation was detected on the virus-infected right FP-S1 region (Fig.3a). Additionally, the BOLD map on the projected left FP-S1 along cortical depth clearly indicates contralateral activation (Fig.3a). The average BOLD map (Fig.3b) and time courses (Fig.3c) to each epoch of the left FP-S1 region show strong positive and negative BOLD responses in L2/3 and L5, indicating that activation in the left hemisphere was most possibly induced via corpus callosum by interhemispheric projections originating from the right hemisphere. The tSNR for BOLD activation in both hemispheres are dramatically increased, showing the inductive coil is up to 3-fold more sensitive than our previous results detected with the surface coil, particularly on the right hemisphere with inserted fiber (Fig.3d). Different to the experiment without optical fiber above, the higher increased sensitivity here clearly demonstrates the inductive coil compensates the signal loss due to experiment complexity for multi-modal fMRI platform.
Conclusion
We present the multi-modal imaging scheme with implanted inductive coil, which yields high-resolution structural and functional images of the rat brain with high signal-to-noise ratio. The smaller sized inductive coil is inductively coupled with larger RF coil allows us to obtain sensory stimulation evoked whole-brain EPI-based fMRI and enhanced focal laminar-specific fMRI signals using a simplified experimental setup. Moreover, it provides the possibility to boost multiregional fMRI sensitivity to investigate the interhemispheric connectivity by embedding inductive coil beneath glue secured optical fiber and projection mirrored cortex on the other hemisphere. This is particularly helpful to study the optogenetic-driven brain connectivity with circuit specificity at multi-modal fMRI platform.Acknowledgements
This research was supported by the internal funding from Max Planck Society, the NIH grants: 1RF1NS113278 and S10 instrument grant (S10 MH124733) to Martinos Center, German Research Foundation Yu215/3-1, BMBF 01GQ1702. This project has received funding from the European Union Framework Programme for Research and Innovation Horizon 2020 (2014-2020) under the Marie Skłodowska-Curie Grant Agreement No.896245.References
1. Yu, X., et al., Sensory and optogenetically driven single-vessel fMRI. Nat Methods, 2016. 13(4): p. 337-40.
2. Lee, J.H., et al., Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature, 2010. 465(7299): p. 788-92.
3. Ryali, S., et al., Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. Neuroimage, 2016. 132: p. 398-405.
4. Lin, P., et al., Optogenetic Functional MRI. J Vis Exp, 2016(110).
5. Bilgen, M., Magnetic resonance microscopy of spinal cord injury in mouse using a miniaturized implantable RF coil. Journal of Neuroscience Methods, 2007. 159(1): p. 93-97.
6. Jordan, C.D., et al., Wireless Resonant Circuits Printed Using Aerosol Jet Deposition for MRI Catheter Tracking. Ieee Transactions on Biomedical Engineering, 2020. 67(3): p. 876-882.
Figures