0128
Forward-Fourier Motion-Corrected Reconstruction for Free-Breathing Liver DCE-MRI1Washington University in St. Louis, Saint Louis, MO, United States
Synopsis
Dynamic contrast-enhanced MRI (DCE-MRI) of the liver offers structural and functional information for assessing the contrast uptake visually. However, respiratory motion and the requirement of high temporal resolution make it difficult to generate high-quality DCE-MRI. In this study, we proposed a novel forward-Fourier motion-corrected reconstruction utilizing deep learning based 3D motion information on severely undersampled DCE-MRI. With no need to use view-sharing or DCE contrast smoothness constraint, this approach avoids enhancement spillover from adjacent DCE contrasts and reconstructs high-quality motion-free DCE images with reduced artifacts and enhanced sharpness.
Introduction
Dynamic contrast-enhanced MRI (DCE-MRI) with high temporal resolution has been used widely for clinical diagnosis to differentiate between benign and malignant lesions.1 Respiratory motion is a major source of artifacts for DCE images of the liver. Clinical standard DCE is acquired during breath-holding. However, many patients fail to comply with breath-holding instructions, often due to comorbid conditions affecting their respiratory or mental statuses. Therefore, free-breathing DCE-MRI techniques have been proposed to minimize respiratory motion artifacts.2–5 View-sharing or compressed sensing (CS) with smoothness constrains across adjacent DCE contrasts has been proposed to reduce reconstruction artifacts.6,7 However, these techniques may lead to contrast enhancement spill-over. This study proposed an iterative forward-Fourier motion-corrected (FF MoCo) reconstruction that incorporates 3D motion vector fields (MVFs) derived from a deep learning network (Phase2Phase) reconstructed 4D MRI. 3D motion-corrected DCE images were generated independently for each DCE contrast.Methods
CAPTURE8, a self-navigated respiratory motion detection MR sequence, was utilized to acquire free-breathing DCE images before, during, and after contrast injection for a total acquisition time of 6 minutes from four patients (3 liver metastases and 1 non-oncologic patients) using a Siemens 3T MRI scanner. Eovist or Dotarem was injected 30 seconds after the start of the CAPTURE scan. The acquisition parameters were as follows: TE =1.32ms-1.64ms, TR=2.82-3.5ms, FOV=380-450mm2, voxel size=1.25x1.25x3mm3 or 1.4x1.4x3mm3, 64-80 slices, 3200-4000 radial spokes.The continuous free-breathing DCE images were first divided into separate DCE contrasts with a time interval of 20 seconds. Within each DCE contrast, the k-space data were binned into 5 respiratory phases and three methods were used to reconstruct 4D respiratory motion-resolved images: (1) MCNUFFT9, (2) CS9, (3) Phase2Phase (P2P)10, a deep learning reconstruction. P2P network was trained without a high-quality ground truth target.11 Non-linear registration was performed to register all respiratory phases to the end-of-expiration phase to obtain 3D deformable MVFs for each of these reconstructions.
Next, a FF MoCo algorithm is used to obtain the final 3D dynamic DCE-MRI for each DCE contrast. Motion correction is carried out iteratively during image reconstruction. The objective function is shown below:
$$
I(x,y,z)=\min_I\sum_t\sum_i\left[E_{t,i}I-K_{t,i}\right]_2^2
$$
where the encoding operator Et,i=FtCiMt ; Ft is the forward Fourier (NUFFT) operator for each respiratory phase t; Ci is the coil sensitivity for Coil i; Mt are the MVFs (from CS, MCNUFFT or P2P reconstruction) deforming the image from respiratory Phase 1 to Phase t; I is the 3D motion-corrected image for each DCE contrast; Kt,i is the acquired k-space data for respiratory Phase t and Coil i.
The FF MoCo reconstruction was repeated for each DCE contrast, resulting in a total of 17 DCE contrasts with a temporal resolution of 20 seconds per DCE contrast. For comparison, 3D spatially motion-corrected (spatial MoCo) DCE images were also reconstructed. In spatial MoCo, 4D motion-resolved MCNUFFT of 5 phases were registered to the end-of-expiration phase and then averaged to produce the final 3D DCE-MRI.
Moreover, we also reconstructed DCE-MRI by FF MoCo with P2P MVF using a temporal resolution of 10 seconds on a patient to test higher temporal resolution.
Results
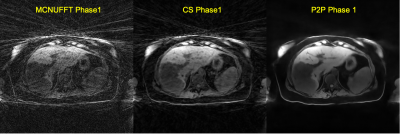
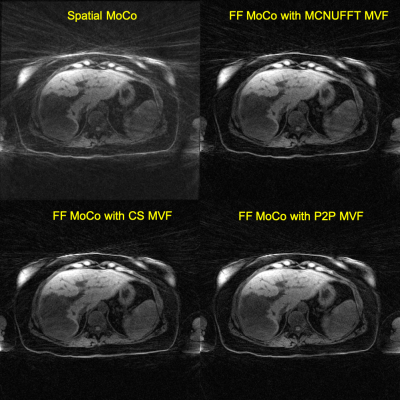
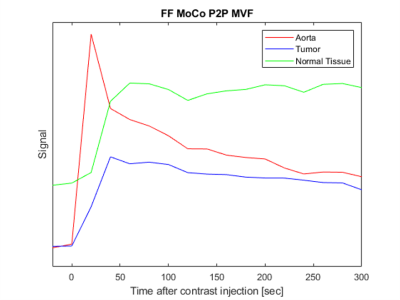
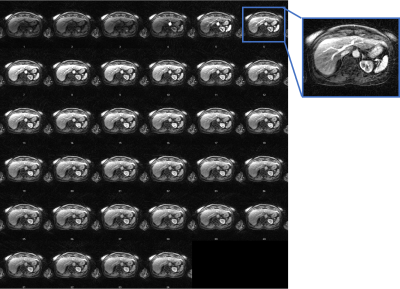
Figure 1 shows sample 4D reconstructed Phase 1 images by MCNUFFT, CS and P2P at Contrast 1 (pre-injection). 4D MCNUFFT here contains severe streaking artifacts; CS reduced but did not eliminate streaking artifacts; P2P has the least amount of artifacts. Figure 2 shows reconstructed DCE Contrast 1 images (pre-injection) on the same patient with liver metastases by Spatial MoCo and FF MoCo (MCNUFFT, CS, or P2P MVF). The results demonstrate that FF MoCo with P2P MVF clearly has the least amount of artifacts. Figure 3 shows FF MoCo P2P MVF images across all 17 DCE contrasts on this patient. These dynamic contrasts allow radiologists to visualize various DCE contrasts, including arterial, portal venous, transitional, and 5-min delay phase from a single free-breathing continuous DCE scan. Figure 4 shows the dynamic contrast enhancement curves on FF MoCo with P2P MVF at aorta, tumor and normal tissues. Eovist uptake curves on FF MoCo P2P MVF exhibit different characteristic uptake patterns. The DCE images reconstructed with a high temporal resolution of 10 seconds also have reasonable quality (Figure 5).Discussion and Conclusion
We proposed an iterative FF MoCo algorithm that incorporates MVFs derived from P2P images to reconstruct high-temporal motion-corrected DCE-MRI. It reconstructed sharp and high-contrast motion-corrected images with fewer artifacts from severely undersampled data – only 4% or 8% of the Nyquist sampling requirement was met in the FF MoCo reconstruction for a temporal resolution of 10 or 20 seconds, respectively.12 Additionally, our method allows for free-breathing continuous DCE scan, with no need for breath hold or a test bolus to determine the exact timing of arterial, portal venous and transitional phases.Furthermore, our method prevents potential contrast enhancement spillover from adjacent DCE contrasts in the view-sharing or CS methods. MVFs were derived using very sparsely sampled data based on the P2P reconstruction. P2P outperforms CS in terms of both the image quality and the speed. CS took a total of 30 hours to reconstruct 17 dynamic contrasts using an Intel Xeon E5-2690v4-Broadwell-EP-2.6-Ghz-CPU, whereas a trained P2P network took only 2 minutes to reconstruct the 4D MR data with one NVIDIA-Tesla-P100-GPU.
Acknowledgements
Funding support for P2P development is provided by Siemens Healthineers.References
1. Choyke, P. L., Dwyer, A. J. & Knopp, M. v. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 17, 509–520 (2003).
2. Cruz, G., Atkinson, D., Buerger, C., Schaeffter, T. & Prieto, C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine 75, 1484–1498 (2016).
3. Reiner, C. S. et al. Contrast-enhanced free-breathing 3D T1-weighted gradient-echo sequence for hepatobiliary MRI in patients with breath-holding difficulties. European radiology 23, 3087–3093 (2013).
4. Zhang, T. et al. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. Journal of Magnetic Resonance Imaging 41, 460–473 (2015).
5. Ippoliti, M. et al. 3D nonrigid motion correction for quantitative assessment of hepatic lesions in DCE-MRI. Magnetic resonance in medicine 82, 1753–1766 (2019).
6. Le, Y., Kroeker, R., Kipfer, H. D. & Lin, C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. Journal of Magnetic Resonance Imaging 36, 483–491 (2012).
7. Levine, E., Daniel, B., Vasanawala, S., Hargreaves, B. & Saranathan, M. 3D Cartesian MRI with compressed sensing and variable view sharing using complementary poisson-disc sampling. Magnetic resonance in medicine 77, 1774–1785 (2017).
8. Eldeniz, C. et al. CAPTURE: Consistently Acquired Projections for Tuned and Robust Estimation: A Self-Navigated Respiratory Motion Correction Approach. Investigative radiology 53, 293–305 (2018).
9. Fessler, J. A. & Sutton, B. P. Nonuniform fast Fourier transforms using min-max interpolation. IEEE transactions on signal processing 51, 560–574 (2003).
10. Eldeniz, C. et al. Phase2Phase: Reconstruction of free-breathing MRI into multiple respiratory phases using deep learning without a ground truth. in Proc Intl Soc Magn Reson Med 28 0807 (Proc Intl Soc Magn Reson Med 28, 2020).
11. Lehtinen, J. et al. Noise2noise: Learning image restoration without clean data. arXiv preprint arXiv:1803.04189 (2018).
12. Liang, Z.-P. & Lauterbur, P. C. Principles of magnetic resonance imaging: a signal processing perspective. (SPIE Optical Engineering Press, 2000).
Figures