0124
Separable motion estimation and correction for 2D TSE imaging using a rapid 3D volumetric scout acquisition1Siemens Healthcare GmbH, Erlangen, Germany, 2Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Department of Radiology, Stanford, Stanford, CA, United States
Synopsis
SAMER is a navigation-free retrospective motion-correction technique which achieves rapid motion estimation using an ultra-fast, low-resolution scout scan as an image prior. In this work, the SAMER framework is extended to 3D volumetric reconstructions of 2D TSE imaging data. The optimized 3D volumetric scout scan is combined with a distributed 2D TSE slice ordering for fully separable motion estimation with negligible added scan time. The motion correction performance was evaluated in-vivo for representative motion trajectories and compatibility to highly accelerated Simultaneous Multi-Slice acquisitions is demonstrated.
Introduction
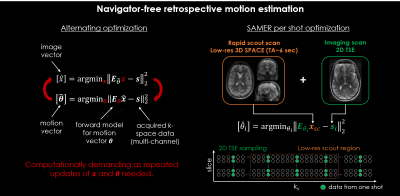
Navigator-free retrospective motion correction techniques [1]–[4] often solve a coupled optimization problem (Fig. 1) where the data-consistency error of a SENSE+motion model is minimized with respect to the unknown motion parameters and an image estimate “jointly”. This poses a computationally demanding non-convex inverse problem with several hundred temporal motion parameters and millions of imaging voxels. The recently proposed SAMER technique [5] speeds-up the motion estimation by using an ultra-fast, low-resolution scout scan as an image prior. This strategy decouples motion estimation from the image reconstruction and avoids the computationally demanding repeated updates of an image estimate (Fig. 1). In addition, the method decouples the motion states themselves leading to a highly scalable computational solution.Here, we extend SAMER from 3D volumetric imaging [5] to 3D volumetric reconstructions of 2D TSE imaging data (including clinically standard slice gaps). A 3D volumetric SPACE scout scan was optimized to minimize additional scan time while maintaining contrast. The 2D TSE slice ordering was improved to increase the temporal resolution of the motion estimation while maintaining the clinically desired contrast. The motion correction performance of our technique was evaluated in-vivo for representative motion trajectories and compatibility to highly accelerated Simultaneous Multi-Slice (SMS) acquisitions [6] is demonstrated.
Methods
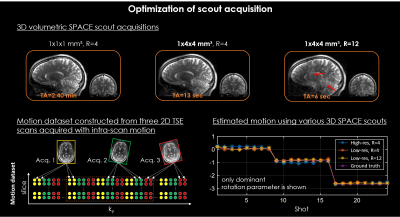
Optimization of scout acquisition:The scout acquisition was optimized to achieve accurate motion estimation with minimal added scan time. We used a prototype 3D SPACE sequence (3D TSE) as the scout, as it provides volumetric imaging data without having to compensate for slice gaps to improve accuracy. To minimize the scan time of the scout we evaluated the motion estimation robustness when the 3D SPACE data was acquired at different spatial resolutions and parallel imaging accelerations. For these analyses, a simulated motion dataset was constructed from three motion-free 2D TSE scans that contained only intra-scan motion (Fig. 2). Ground truth parameters were obtained from image space registrations. All scans were acquired on a 3T system (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. The 3D volumetric coil sensitivity maps were computed from an optimized 2 sec external GRE reference scan using ESPIRiT [7].
Optimization of 2D TSE slice ordering:
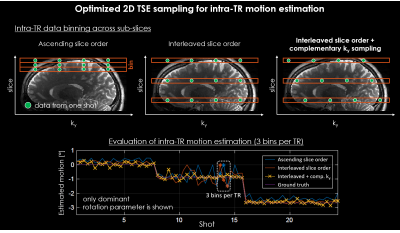
The 2D TSE slice ordering was optimized to allow for higher temporal estimation of the motion. Standard T2w TSE acquisitions often use long TRs (~6 sec) which is insufficient to capture typical patient motion. Higher temporal resolution can be achieved by intra-TR data binning. However, the smaller amount of slice-ky data used can lead to decreased motion estimation accuracy. We introduce an interleaved slice ordering scheme with complementary ky sampling and compare the robustness of the motion estimation to a standard sampling pattern. The simulated motion data described above (Fig. 2) was used and intra-TR data binning was performed by grouping the data from each TR into three motion bins.
In vivo motion experiments:
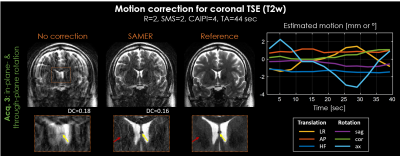
The motion correction performance of our approach was evaluated in-vivo for axial T2w TSE with supervised subject motion (in-plane and/or through-plane). In addition, the compatibility to highly accelerated Simultaneous Multi-Slice acquisitions (R=2, MB=2) with CAIPIRINHA sampling [8] was demonstrated. In all motion experiments, a R=12-fold accelerated 3D SPACE scout (TA=6 sec, Fig. 2) and an interleaved 2D TSE ordering scheme with complementary ky sampling (Fig. 3) were used to estimate 3D motion every two seconds (three motion bins per TR).
Results
Accurate estimation of 3D motion within 2D TSE imaging data was achieved using an optimized, six-second 3D volumetric SPACE scout acquired at low spatial resolution and high acceleration (Fig. 2).Intra-TR data binning with standard ascending slice ordering yielded inaccurate motion estimation, as opposed to an interleaved slice ordering with complementary ky sampling (Fig. 3).
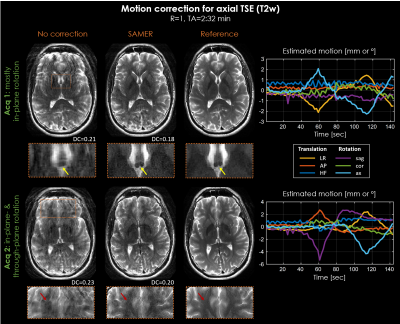
Reduction of motion artifacts was achieved for all in-vivo acquisitions (Fig. 4+5). SAMER improved delineation of fine anatomical structures, e.g., vessels (yellow arrows), and resulted in a decrease of the data-consistency-error (DC). However, despite a full 3D reconstruction, residual motion artifacts and some loss of spatial resolution (red arrows) were observed, especially, in the case of through-plane rotation.
Discussion
In this work, we demonstrated 3D motion artifact mitigation for standard clinical 2D TSE imaging where the use of a rapid 6 sec 3D volumetric scout enabled highly efficient and separable motion estimation and correction. We evaluated the motion mitigation performance in-vivo and observed robust artifact reduction across a variety of motion trajectories.Intra-TR data binning was used to increase the temporal resolution of the 3D motion estimation. Here, three bins per TR were used which led to a three-fold improvement in temporal resolution but with proportional increases to the reconstruction. Dynamically binning the data is likely to improve the trade-off of motion correction accuracy and reconstruction time.
Through-plane interpolation across thick slices, spin history, and slice gaps caused residual motion artifacts despite the use of a full 3D reconstruction model. Future work will investigate the use of data/slice over-sampling [4] and/or machine learning [10] to compensate for missing or degraded data. Moreover, SMS can allow for a flexible trade-off between additional scan time and image quality.
Acknowledgements
No acknowledgement found.References
[1] M. W. Haskell, S. F. Cauley, and L. L. Wald, “TArgeted Motion Estimation and Reduction (TAMER): Data consistency based motion mitigation for mri using a reduced model joint optimization,” IEEE Trans. Med. Imaging, vol. 37, no. 5, pp. 1253–1265, 2018.
[2] A. Loktyushin, H. Nickisch, R. Pohmann, and B. Schölkopf, “Blind multirigid retrospective motion correction of MR images,” Magn. Reson. Med., vol. 73, no. 4, pp. 1457–1468, 2015.
[3] L. Cordero-Grande, R. P. A. G. Teixeira, E. J. Hughes, J. Hutter, A. N. Price, and J. V. Hajnal, “Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction,” IEEE Trans. Comput. Imaging, vol. 2, no. 3, pp. 266–280, 2016.
[4] L. Cordero-Grande, E. J. Hughes, J. Hutter, A. N. Price, and J. V. Hajnal, “Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging,” Magn. Reson. Med., vol. 79, no. 3, pp. 1365–1376, 2018.
[5] D. Polak, S. Cauley, D. Splitthoff, P. Bachert, and K. Setsompop, “Scout Acquisition enables rapid Motion Estimation (SAME) for fully separable retrospective motion mitigation,” in Proceedings of ISMRM 28th Annual Meeting, 2020.
[6] D. J. Larkman, J. V. Hajnal, A. H. Herlihy, G. A. Coutts, I. R. Young, and G. Ehnholm, “Use of multicoil arrays for separation of signal from multiple slices simultaneously excited,” J. Magn. Reson. Imaging, 2001.
[7] M. Uecker et al., “ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA,” Magn. Reson. Med., vol. 71, no. 3, pp. 990–1001, Mar. 2014.
[8] F. A. Breuer, M. Blaimer, R. M. Heidemann, M. F. Mueller, M. A. Griswold, and P. M. Jakob, “Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging,” Magn. Reson. Med., vol. 53, no. 3, pp. 684–691, 2005.
[9] D. Polak et al., “Joint multi-contrast variational network reconstruction (jVN) with application to rapid 2D and 3D imaging,” Magn. Reson. Med., 2020.
[10] M. W. Haskell et al., “Network Accelerated Motion Estimation and Reduction (NAMER): Convolutional neural network guided retrospective motion correction using a separable motion model,” Magn. Reson. Med., vol. 82, no. 4, pp. 1452–1461, 2019.
Figures