0123
PET/MR respiratory motion gating for free
Florian Wiesinger1, Timothy Deller2, Floris Jansen2, Jose de Arcos Rodriguez1, Ronny R Buechel3, Philipp A Kaufmann3, and Edwin EGW ter Voert3
1GE Healthcare, Munich, Germany, 2GE Healthcare, Waukesha, WI, United States, 3Department of Nuclear Medicine, University Hospital Zurich, Zurich, Switzerland
1GE Healthcare, Munich, Germany, 2GE Healthcare, Waukesha, WI, United States, 3Department of Nuclear Medicine, University Hospital Zurich, Zurich, Switzerland
Synopsis
Respiratory motion correction is a long-standing problem in hybrid PET/MR imaging with many partial solutions. Here we present a novel method based on extracting respiratory motion information from the PET data using the ultra-fast listmode reconstruction framework. Doing so a highly accurate respiratory waveform derived from the inside of the body (i.e. lung liver interface) is obtained for free without requiring an extra motion sensor or complicating the PET/MR imaging workflow.
Introduction
PET/MR is a hybrid imaging modality allowing simultaneous acquisition of MR and PET information, providing unique opportunities in terms of joint image reconstruction and motion correction. In current methods often MR is used to extract motion information which is then used for PET and/or MR motion gating and/or correction. This requires dedicated MR sequences and limits the availability of motion information to the duration of the MR scan. Alternatively, also auxiliary motion sensors (respiratory belt, optical camera, …) are used. However, these are typically expensive, complicate workflow, require cross-calibration, might drift, or pick-up additional motions. Recently Spangler-Bickell et al1 introduced a PET reconstruction framework which reconstructs the PET listmode raw data into dynamic PET frames with high temporal resolution of ≤ 1 sec (in addition to standard PET images). Here we describe the adaptation of this method for MR and PET respiratory motion correction.Methods
All scans were performed on a GE SIGNA PET/MR scanner (GE Healthcare, Chicago, IL) using either [13N]-Ammonia or [18F]-FDG PET tracers. The PET/MR patient imaging (with signed informed consent by the patients and ethics approval by the local ethics commission) focused on the chest region and was performed in free breathing. PET data were collected continuously throughout the duration of the bed position and recorded sequentially in the listmode file. The ultra-fast listmode reconstruction framework1 was used to reconstruct temporal PET frames at 1.0 sec temporal resolution for the whole duration of the PET bed position. A respiratory waveform (RW) was then extracted from the high-temporal resolution PET images using either #1) a cylindrical pencil beam navigator across the lung-liver interface (RWNAV), or #2) Principal Component Analysis (PCA) of the dominant motion component (RWPCA). The PET-derived respiratory waveform was then used for retrospective soft-gating of both MR and PET data. Simultaneous to the PET also MR scanning was performed including six repetitions of a 3D radial Zero TE (ZTE) sequence for structural lunging imaging2 [FOV=400x400x288mm3, res=1.6x1.6x1.6mm3, FA=2deg, BW=±62.5kHz, time=71sec]. The ZTE sequence contained multiple sequential repetitions (m) to allow soft-gating of the acquired data into different respiratory phases (n) according to: wn,m=exp[-(bm-refn)2/α2]; with bm the respiratory amplitude of the mth spoke relative to a reference position (refn) and α defining a soft acceptance window. The soft-gated k-space data were then reconstructed into a dynamic 4D ZTE image using 3D gridding.Results
The animated Figures 1 and 2 show example results of a patient injected with [13N]-Ammonia (injected dose 200-600 MBq dependent on BMI and stress/rest) where the ZTE imaging started ~8min after the PET tracer injection. Figure 1 illustrates the dynamic PET images at 1 sec temporal resolution (top). The normalized respiratory waveforms (bottom) obtained using the pencil beam navigator and the PCA method demonstrate excellent agreement. The position and duration of the six ZTE acquisitions are indicated as black thick lines along the time axis. This patient demonstrates a deep, regular diaphragmatic breathing pattern. Figure 2 illustrates corresponding ZTE images. Because of the deep diaphragmatic breathing the uncorrected images (left) show strong motion blurring especially at the lung-liver interface. Soft-gated respiratory binning (7 phases, 2nd column) resolves the diaphragmatic breathing cycle into 7 phases. Most of the data are acquired during end-expiration (3rd column) which also provides the sharpest image. Its Maximum Intensity Project (MIP, right) depicts the vascular anatomy and lung lesions in fine detail. The animated Figures 3 and 4 illustrate a patient injected with [18F]-FDG (injected dose = 250 MBq) where the ZTE imaging started ~100min after the PET tracer injection. Again, the navigator and PCA-based respiratory waveforms (Fig. 3) illustrate excellent agreement. Because of the shallow chest breathing the uncorrected ZTE (Fig. 4, left) shows only benign motion blurring which is also demonstrated by the respiratory binning (7 phases, 2nd column). The end-expiratory phase ZTE image (3rd column) and its MIP (right) are illustrated as well.Discussion
Respiratory motion correction is a long-standing problem in hybrid PET/MR imaging with many partial solutions. Here we present a novel method based on extracting respiratory motion information from the PET data using the ultra-fast listmode reconstruction framework. Doing so a highly accurate respiratory waveform derived from the inside of the body (i.e. lung liver interface) is obtained for free without requiring an extra motion sensor or complicating the PET/MR imaging workflow. The obtained respiratory waveform can then be used to soft-gate PET and MR images to the very same breathing phase and thereby assure perfect geometric alignment in presence of respiratory motion.Acknowledgements
No acknowledgement found.References
- Spangler-Bickell et al (2020). Ultra-Fast List-Mode Reconstruction of Short PET Frames and Example Applications. Journal of Nuclear Medicine.
- Gibiino et al (2015). Free-breathing, zero-TE MR lung imaging. Magnetic Resonance Materials in Physics, Biology and Medicine, 28(3), 207-215.
Figures
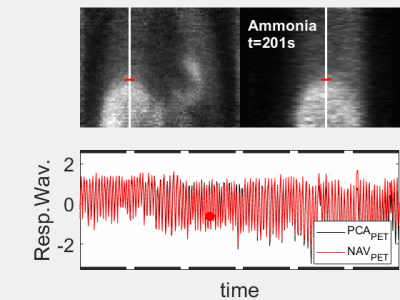
Figure 1 (animated): High temporal-framerate PET
reconstruction (Δt=1s) in the
transient phase ~8mins after the injection of the Ammonia PET tracer.
Corresponding respiratory waveforms (bottom) obtained using either Principal
Component Analysis (PCA, black) or a pencil beam navigator over the lung-liver
interface (red). This patient
demonstrates a deep, regular diaphragmatic breathing pattern.
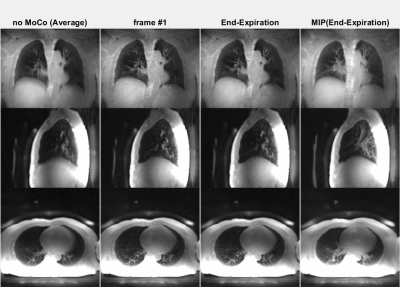
Figure 2 (animated): ZTE lung images corresponding to the Ammonia PET tracer
patient shown in Figure 1. Because of
the deep diaphragmatic breathing the uncorrected (averaged, left) images
show strong motion blurring (especially at the lung-liver interface). Soft-gated
respiratory binning (7 phases, 2nd column) resolves the diaphragmatic
breathing cycle into 7 phases. Most
of the data are acquired in the end-expiratory phase (3rd column) also
providing the sharpest image. Its
Maximum Intensity Project (MIP, right) depicts the vascular anatomy and lung
lesions in fine detail.
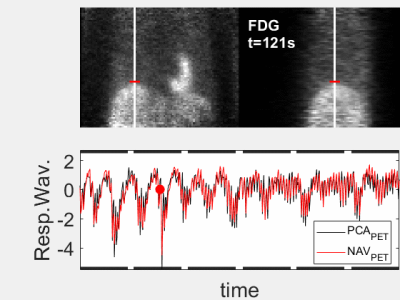
Figure 3 (animated): High temporal-framerate PET
reconstruction (Δt=1s) after
~100min update time following the injection of the FDG PET tracer.
Corresponding respiratory waveforms (bottom) obtained using either Principal
Component Analysis (PCA, black) or a pencil beam navigator over the lung-liver
interface (red). This patient
demonstrates a shallow, irregular chest breathing pattern.
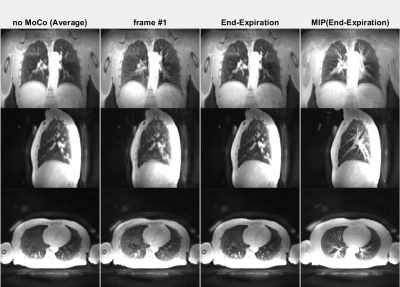
Figure 4 (animated): ZTE lung images corresponding to the FDG PET tracer patient shown in
Figure 3. Because of the shallow
chest breathing the uncorrected (averaged, left) images show benign motion
blurring. Soft-gated respiratory binning
(7 phases, 2nd column) resolves the chest breathing cycle into 7
phases. Most of the data are acquired in
the end-expiratory phase (3rd column) also providing the sharpest
image. Its Maximum Intensity Project
(MIP, right) depicts the vascular anatomy and lung lesions in fine detail.