0120
MERLIN: Motion Insensitive Silent Neuroimaging1Neuroimaging, Institute of Psychiatry, Psychology, and Neuroscience, King's College London, London, United Kingdom, 2ASL Europe, GE Healthcare, Munich, Germany
Synopsis
In this work we present MERLIN (Motion Elimination in Radial acquisition Leveraging Interleaved Navigators): a new method for silent, motion insensitive, MRI using self-navigated zero echo time (ZTE) imaging. Using T1w ZTE neuroimaging as an example, we demonstrate that MERLIN can correct for rigid body motion and markedly improve image quality. Such a silent and motion insensitive neuroimaging protocol can save time and money in both clinical and research settings.
Introduction
Acoustic noise and patient motion are two main obstacles in diagnostic MRI, causing additional stress for the patient, complications for the medical staff and extra cost for the healthcare system1,2. Each issue has previously been investigated separately: methods to combat motion include navigator echoes3, self-navigation4 and external tracking devices5, while acoustic noise can be reduced using smooth gradient waveforms6 or Zero Echo Time (ZTE) pulse sequences7,8.In this work we address both issues, by combining silent ZTE with a self-navigated phyllotaxis trajectory for retrospective motion correction. We call this method MERLIN for Motion Elimination in Radial acquisition Leveraging Interleaved Navigators.
Methods
ZTE sequences acquire data along radial-out spokes during the free induction decay. With a TR on the order of ≈1-3ms, flip angles 1-5°, and RF spoiling between excitations, ZTE sequences can be treated as spoiled gradient echo sequences9, making them suitable for magnetisation preparation, like MPRAGE10.MERLIN adds two components to a standard ZTE pulse sequence: an interleaved k-space trajectory for self-navigation, and a retrospective motion correction framework. Here we describe an example using IR-ZTE for high-resolution T1w neuroimaging.
A single healthy volunteer was scanned on a 3T GE MR750 (GE Healthcare, Chicago, IL), after providing written consent. IR-ZTE data were acquired with readout BW=±31.25 kHz, FOV=192x192x192mm3, resolution=1x1x1mm3, TI=450ms, FA=3°, TR=1.8ms, 384 spokes per readout segment, 108,288 spokes in total, and acquisition time=5:42min. IR-ZTE images were reconstructed with and without motion correction, using an iterative TGV-regularized gridding reconstruction (λ=0.00001)11. All ZTE image reconstruction was performed using the RIESLING toolbox (https://github.com/spinicist/riesling).
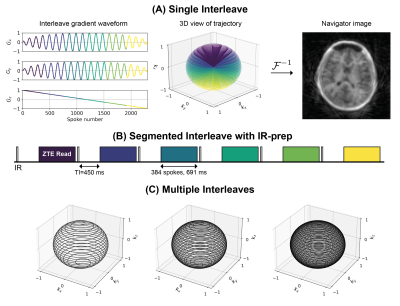
A Self-navigated 3D Radial Trajectory
To maintain a silent acquisition, the change in gradient amplitude between excitations has to be small. The spiral phyllotaxis trajectory12,13 can be formulated to provide self-navigated high-resolution imaging while still requiring only minimal gradient switching for silent operation. Our trajectory was designed to have 47 interleaves (Nint), each with 2304 spokes (Nspi). An interleave is defined as a single spiral path covering k-space from top to bottom (Figure 1A). The azimuthal φi,k and polar θi,k angles for spoke i in interleave k are given by
$$
\phi_{i,k}=i\phi_G\cdot{F(s)}+k\phi_G, \quad \theta_{i,k}=\cos^{-1}\left(1-\frac{2\cdot{N}_{int}\cdot{i}}{N-1}-\frac{2\cdot{k}}{N-1}\right)
$$
$$
i=0...N_{spi}-1,\quad{k}=0...N_{int}-1,\quad{N}=N_{spi}\cdot{N_{int}}
$$
where φG is the golden angle, F(·) is the Fibonacci sequence, and s is the smoothness factor, here s=10 which results in an azimuthal step of 2.9° (mod(F(10)·φG,2π)). The interleaves were divided into 6 segments (Nseg) to allow for IR preparation (Figure 1B). Subsequent interleaves are rotated by the golden angle relative to each other to produce pseudo-random, uniform, k-space coverage (Figure 1C).
Motion Correction
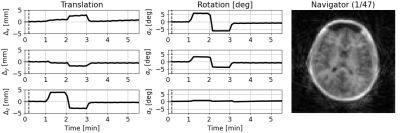
Interleaves were reconstructed using CG-SENSE14 at a lower-resolution (3x3x3mm3, effective R=5.6) to produce motion navigators. These were co-registered using a 3D rigid body transformation (VersorRigid3DTransform) implemented in ITK15. Estimated translations (Δx, Δy, Δz) were corrected in k-space by applying a phase ramp, and rotations (αx, αy, αz) were corrected by rotating the trajectory16, producing a corrected k-space dataset for reconstruction with TGV as described above.
Motion Experiment
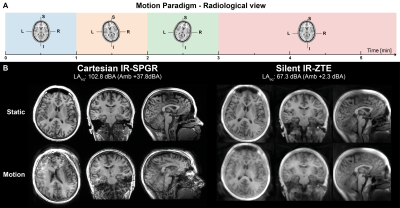
Two acquisitions were performed, one without and one with motion; for the latter, the subject was asked to repeat a pattern of rotating the head in the coil according to instructions (Figure 2A). For comparison, a conventional, 3D Cartesian IR-SPGR scan was acquired, also with TI=450ms, acquisition time=5:36, also with and without motion. Acoustic noise measurements were performed for a period of 30s in the centre of the bore.
Results
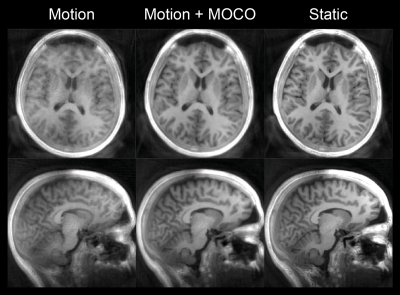
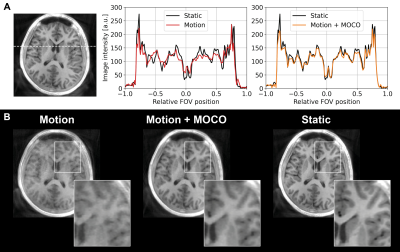
Figure 2B shows IR-SPGR compared to IR-ZTE reconstructed without motion correction. The repeated sampling of centre of k-space in IR-ZTE results in improved robustness to motion, but motion nevertheless resulted in significant blurring. The acoustic noise from IR-ZTE was only 2.3dBA over the ambient compared to 37.8dBA above with IR-SPGR.The estimated motion parameters shown in the animated Figure 3 clearly follows the movement pattern that the volunteer was instructed to perform, with step-like changes at 1, 2, and 3 minutes into the scan. Figure 4 shows the IR-ZTE data with motion correction, showing markedly improved image quality. Figure 5A shows a line-profile in the axial-plane which clearly shows how the image intensity in the corrected image closely follows the static image. The magnified area in Figure 5B shows how the white and gray matter boundaries are better resolved with motion correction.
Discussion and Conclusion
In this work we have presented MERLIN, a framework for self-navigated motion correction using silent ZTE. MERLIN only requires a simple modification to the k-space trajectory typically used in ZTE imaging and is also consistent with other types of contrast preparation (e.g., T1, T2, DWI, MRA, etc.).To maintain a near-silent acquisition, the gradient steps between spokes have to be kept small, which limits the trajectory to spiral-like patterns. With the IR-ZTE protocol used here, 40% of the acquisition time is spent on the inversion time. This prolongs the time required for each navigator, here about to ~7s. The duration of each navigator can be reduced by using fewer spokes, but at the expense of faster gradient switching and higher acoustic noise, as well as reduced SNR in each navigator. Further work will focus on reducing the time required for each navigator to improve the temporal resolution of the motion estimates.
Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z], the National Institute for Health Research (NIHR) Wellcome Trust King’s Clinical Research Facility at King's College Hospital, and GE Healthcare.References
1. Andre, J. B. et al. Toward Quantifying the Prevalence, Severity, and Cost Associated With Patient Motion During Clinical MR Examinations. J. Am. Coll. Radiol. 12, 689–695 (2015).
2. Sartoretti, E. et al. Impact of Acoustic Noise Reduction on Patient Experience in Routine Clinical Magnetic Resonance Imaging. Acad. Radiol. 0, 1–8 (2020).
3. White, N. et al. PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking. Magn. Reson. Med. 63, 91–105 (2010).
4. Pipe, J. G. Motion Correction With PROPELLER MRI: Application to Head Motion and Free-Breathing Cardiac Imaging. Magn. Reson. Med. 42, 963–969 (1999).
5. Zaitsev, M., Dold, C., Sakas, G., Hennig, J. & Speck, O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 31, 1038–1050 (2006).
6. Hennel, F. Fast spin echo and fast gradient echo MRI with low acoustic noise. J. Magn. Reson. imaging 13, 960–6 (2001).
7. Madio, D. P. & Lowe, I. J. Ultra-Fast Imaging Using Low Flip Angles and FIDs. Magn. Reson. Med. 34, 525–529 (1995).
8. Weiger, M. & Pruessmann, K. P. MRI with Zero Echo Time. eMagRes 1, 311–322 (2012).
9. Ljungberg, E. et al. Silent T1 mapping using the variable flip angle method with B1 correction. Magn. Reson. Med. 84, 813–824 (2020).
10. Costagli, M. et al. Assessment of Silent T1-weighted head imaging at 7 T. Eur. Radiol. 26, 1879–1888 (2016).
11. Knoll, F., Bredies, K., Pock, T. & Stollberger, R. Second order total generalized variation (TGV) for MRI. Magn. Reson. Med. 65, 480–491 (2011).
12. Swinbank, R. & Purser, R. J. Fibonacci grids: A novel approach to global modelling. Q. J. R. Meteorol. Soc. 132, 1769–1793 (2006).
13. Piccini, D., Littmann, A., Nielles-Vallespin, S. & Zenge, M. O. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 66, 1049–1056 (2011).
14. Pruessmann, K. P., Weiger, M., Börnert, P. & Boesiger, P. Advances in Sensitivity Encoding With Arbitrary k-Space Trajectories. Magn. Reson. Med. 46, 638–651 (2001).
15. Mccormick, M., Liu, X., Jomier, J., Marion, C. & Ibanez, L. ITK: Enabling reproducible research and open science. Front. Neuroinform. 8, 1–11 (2014).
16. Maas, L. C., Frederick, B. D. B. & Renshaw, P. F. Decoupled automated rotational and translational registration for functional MRI time series data: The DART registration algorithm. Magn. Reson. Med. 37, 131–139 (1997).
Figures