0117
MRI of [2-13C]Lactate without J-coupling artifacts
Keshav Datta1 and Daniel Spielman1
1Department of Radiology, Stanford University, Stanford, CA, United States
1Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Metabolic imaging using hyperpolarized [2-13C]Pyruvate has the potential to simultaneously probe glycolysis and Kreb’s cycle, but one of its major limitations is the difficulty in imaging [2-13C]Lactate. The peak-splitting induced by the J-coupling between the C2 carbon and its attached proton causes ghosting and blurring artifacts, depending on the k-space trajectory. We propose two novel techniques, the first a two-shot approach combining in-phase and quadrature images acquired at echo times differing by 1/2J and the second a single-shot method employing a highly narrowband radiofrequency excitation pulse that images a single peak from the doublet, to resolve the J-modulated artifacts.
1.1 Introduction
[2-13C]Pyruvate (Pyr) is an attractive choice for hyperpolarized imaging with first human studies reported recently1. The 13C label at the C2 position is both converted to [2-13C]Lactate (Lac) and observable from the tricarboxylic acid (TCA) cycle intermediates citrate, glutamate and acetylcarnitine, thus providing a simultaneous real-time window into glycolysis (GLY) and oxidative phosphorylation (OXPHOS)2,3. However, imaging [2-13C]Lac is challenging due to blurring and ghosting artifacts arising from J-coupling between the 13C2 and the 1H nuclei. We present two techniques based on the idea of quadrature detection to resolve these artifacts. The key contribution of this work is the use of product operator formalism to derive the evolution of coherences in the presence of J-coupling as well as chemical shift to show that the combination of in-phase and anti-phase doublets resolves the peak-splitting during image acquisition. Results from simulations, phantoms and rat brain highlight the utility of this method for simultaneous assessment of GLY and OXPHOS in vivo. While these techniques are presented in the context of hyperpolarized 13C metabolic imaging, they are generally applicable to any spin-1/2 J-coupled spin system. Details can be found in this publication4.1.2 Theory
Quadrature imagingWhen imaging compounds such as [2-13C]Lac, J-coupling between the labeled carbon and attached proton causes a cosine modulation of k-space samples given by $$$S^{'}[k_x(t),k_y(t)]=cos(πJt+φ)S[k_x(t),k_y (t)] $$$ (Fig. 1). Measuring the quadrature component via a second acquisition delayed by 1/2J can compensate for this modulation, resulting in an artifact-free image with complex combination of the two acquisitions:
$$I_{rec} (x,y)=I(x,y)*IFT(cos(πJt+φ))+iI(x,y)*IFT({cos(πJ(t+\frac{1}{2J})+φ) })\qquad$$
$$ =I(x,y)*IFT(cos(πJt+φ))+iI(x,y)*IFT({({sin(πJt+φ)})}\space\space\space$$
$$=I(x,y-Y)e^{iφ }\qquad\qquad\qquad\qquad\qquad\qquad\qquad\qquad\qquad\space\space\space\space [1]$$
where φ represents the phase accumulation due to the delay between excitation and data acquisition. Y is an easily corrected spatial shift given by $$$k_y Y=\frac{J.T_{readout}}{2}$$$.
Narrowband excitation
For a spin-1/2 J-coupled system ($$$\hat{I}$$$≡13C and $$$\hat{S}$$$≡1H), a sufficiently narrow spectrally selective excitation pulse applied at off-resonance frequency $$$Ω_I=πJ$$$ generates the following coherences at the end of the radiofrequency pulse:
$$ \hat{σ}_{RF}=(\frac{1}{2})\hat{I}_z-(\frac{1}{2}) 2\hat{I}_z\hat{S}_z+(\frac{1}{2})\hat{I}_y+(\frac{1}{2})2\hat{I}_y\hat{S}_z\qquad\qquad\qquad [2]$$
During readout, the sum of in-phase doublet from the $$$\hat{I}_y$$$ term and anti-phase doublet from $$$2\hat{I}_y\hat{S}_z$$$ produces a singlet, eliminating all J-coupling associated artifacts (Fig. 2).
1.3 Methods
Simulation & in vitro experiments: A full-density matrix simulator for a coupled 2-spin I-S system in MATLAB (Mathworks Inc.) developed in-house was used to simulate the evolution of coherences. A syringe filled with 10ml of 0.5M [2-13C]Lac (or 3ml of 2.5M 13C Formate), doped with a Gadolinium agent, was placed in a 13C transmit-receive surface coil and imaged using a clinical 3T PET-MR scanner (GE Medical systems). For quadrature imaging, two images whose echo times differed by 1/2J=3.6 ms (J=140Hz for [2-13C]Lac) were acquired using a slice-selective fast echo-planar imaging sequence (gradient echo EPI,FOV=128mm,slice-thickness=20mm,TR=130ms,TE=22.8ms,FA=800,matrix=32x32,receiver BW=41.7kHz,NEX=150). A narrowband spectrally selective radiofrequency excitation pulse (BW=100Hz,slice-thickness=20mm,70Hz off-resonance), followed by a 2D GRE-EPI image acquisition (FOV=128mm,TR=130ms,TE=22.8ms,FA=800,matrix=32x32,receiver BW=41.7kHz,NEX=150) , was used to test the narrowband technique.Hyperpolarization and in vivo imaging of [2-13C]Lac: 54ml of 15.5M [2-13C]Pyruvic acid mixed with 15mM trityl radical was polarized between 3-4 hours in GE SPINLab system and dissolved using 16g solution of 40mM tris buffer,100mg/L EDTA, and 50mM NaCl. After neutralizing with 640ul of 125mM NaOH buffer, 2.5ml of the resulting 100mM pyruvate solution was injected at a rate of 0.25mL/s into a male Wistar rat through a tail-vein catheter and imaged in a clinical GE 3T scanner 20s post injection to maximize the signal from lactate. A narrowband radiofrequency(RF) pulse centered on one of the lactate peaks followed by an EPI readout, with the same parameters as for the phantom, was used to acquire the [2-13C]Lac image from an axial slice through the rat-brain and overlaid on a T1-weighted proton image for anatomical reference.
Reconstruction and post-processing: An empirical scale factor, Acorr, to compensate for the T1 and flip angle related losses, and a relative phase factor ϕcorr to counter the phase accumulation arising from off-resonance effects (offres) were introduced in Eq. [1], i.e., Irec (x,y)=I1 (x,y)+iAcorrI2 (x,y) eiϕcorr. The resulting image was shifted by $$$(\frac{J}{2}+offres )*T_{readout}$$$ pixels in the slower k-space acquisition direction to offset the shift arising from the quadrature reconstruction. In the case of narrowband acquisition, the native scanner EPI reconstruction was used without any further processing.
1.4 Results
Quadrature Imaging (Fig. 3): EPI acquisitions from thermally polarized and hyperpolarized phantoms show the severity of J-modulation artifacts, which are resolved via the complex combination of two acquisitions, one delayed by 1/2J.Narrowband Imaging: Analytically derived and simulated coherences during broadband and narrowband RF excitations are shown in Fig. 4. Data from simulations, [2-13C]Lac and 13C-formate phantoms (Fig. 4), and an in vivo [2-13C]Pyr rat brain study (Fig. 5), demonstrate the effectiveness of the narrowband excitation approach.
1.5 Discussion and Conclusions
The two-shot and single-shot imaging strategies described here utilize the same underlying principle of combining in-phase and quadrature components to recover the signal free of J-coupling artifacts. The two-shot method sequentially acquires the sine and cosine components, whereas the single-shot technique based on use of highly selective RF pulse simultaneously generates in-phase (cosine) and anti-phase (sine) coherences that combine to produce a singlet during imaging. Simulations, in vitro, and in vivo results demonstrate the potential utility of these methods for imaging of [2-13C]Lac in a hyperpolarized [2-13C]Pyr experiment.Acknowledgements
The Lucas Foundation, NIH grants R01EB019018, R01CA176836, P41 EB015891.References
- Chung BT, Chen HY, Gordon J, et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. Journal of Magnetic Resonance. vol. 309, p. 106617, 2019. DOI: https://doi.org/10.1016/j.jmr.2019.106617
- Schroeder MA, Atherton HJ, Ball DR, et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. The FASEB Journal. vol. 23, no. 8, pp. 2529-2538, 2009. DOI: https://doi.org/10.1096/fj.09-129171
- Park JM, Josan S, Grafendorfer T, et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2‐13C]pyruvate. NMR in Biomedicine. vol. 26, no. 10, pp. 1197-1203, 2013. DOI: https://doi.org/10.1002/nbm.2935
- Datta K, Spielman D. MRI of [2-13C]Lactate without J-coupling artifacts. Magn. Reson Med. 85: 1522-1539, 2020. DOI: https://doi.org/10.1002/mrm.28532
Figures
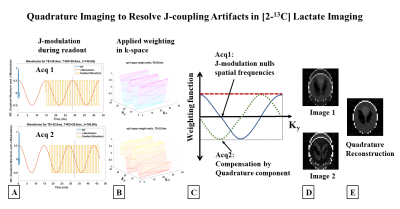
Figure 1 (A) Schematic of an RF pulse followed by gradient
waveform for EPI readout (FOV=128mm, 32x32 matrix, TE=28.4ms, readout=28.8ms)
highlights the evolution of J-modulation during imaging. (B) k-space weighting due
to J-modulation is significant in the slower phase encode direction (ky). (C) Spatial frequencies nulled due to J-coupling
evolution during readout (Acq1), compensated by acquiring a quadrature
component by delaying the acquisition time by 1/2J (Acq2). (D) Complex
combination of these two quadrature components restores the original image, (E).
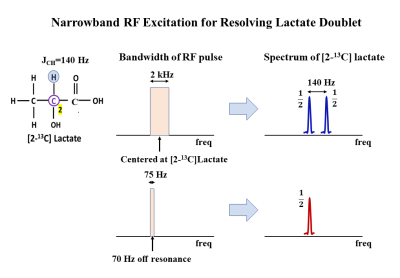
Figure 2 Narrowband excitation for resolving J-coupling
induced doublet in [2-13C]Lac. Evolution of J-coupling during a
short RF pulse (500μs, 2kHz bandwidth) is negligible and hence results
in a doublet during signal collection (top row). A long narrow bandwidth pulse
(13.33ms, 75Hz), J/2 Hz off-resonance (70Hz), on the other hand, generates
in-phase and anti-phase coherences that combine to result in a singlet during
signal acquisition.
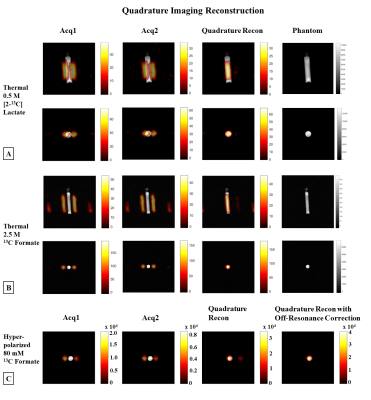
Figure 3
Quadrature imaging. (A) Coronal (1st row) & axial (2nd
row) images from [2-13C]Lac phantom (JCH=140Hz), & (B) 13C-formate
phantom (JCH=192Hz), overlaid on the proton images. The quadrature
reconstruction (3rd column) resolves the ghosting artifacts seen in
Acq1 & Acq2. Proton MRI of the phantom depicts its location (last column). (C)
EPI reconstructions of a 20mm axial slice from a hyperpolarized 13C-formate
phantom overlaid on the proton image. Quadrature reconstruction resolves
ghosting, & the residual artifact is completely removed with a relative phase
correction.
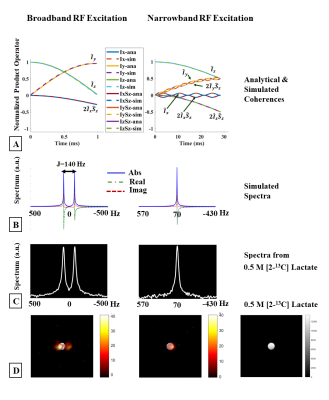
Figure 4
Narrowband excitation. (A) Analytical (solid) and simulated (dashed) evolution
of coherences during a 1ms on-resonance broadband pulse (left) and a 28.5ms,
70Hz off-resonance narrowband RF pulse (right), J=140Hz. Spectra from
simulations, (B), agree very well with (C), spectra acquired from a 0.5M [2-13C]Lac
phantom. The doublet resulting from a short duration RF pulse (left) is
resolved into a singlet when a long-duration narrowband RF pulse is used
(right). (D) EPI images acquired from the phantom after broadband (left) and
narrowband excitation (center).
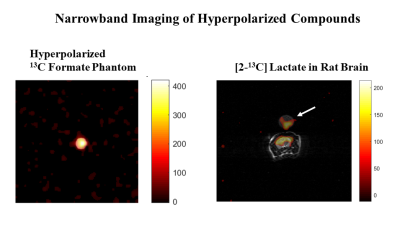
Figure 5 Narrowband imaging. A frequency selective
RF pulse (pulse width 24ms, bandwidth 133Hz, slice thickness 20mm) followed by
an EPI readout (FOV=128mm, TR=130ms, TE=22.8ms, FA=800,
32x32, RBW=41.7kHz) produces artifact-free images of an axial slice of (A)
hyperpolarized 13C-formate phantom (dia. 12.5mm), and (B) in vivo
[2-13C]Lac generated in the brain of a male Wistar rat after an
injection of 2.5 ml of hyperpolarized [2-13C]Pyr. The artifact-free
image of the reference [2-13C]Lac phantom placed on top of the rat
head is also visible (indicated by the arrow).