0116
Simultaneous Multiple Resonance Frequency Imaging (SMURF): Fat‑water imaging using multi‑band principles
Beata Bachrata1,2, Bernhard Strasser1,3, Wolfgang Bogner1, Albrecht Ingo Schmid4, Radim Korinek5, Martin Krššák1,2,6, Siegfried Trattnig1,2, and Simon Daniel Robinson1,7,8
1High Field MR Centre, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Karl Landsteiner Institute for Clinical Molecular MR in Musculoskeletal Imaging, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4High Field MR Centre, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 5Institute of Scientific Instruments of the CAS, Brno, Czech Republic, 6Department of Internal Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna, Vienna, Austria, 7Centre of Advanced Imaging, University of Queensland, St. Lucia, Australia, 8Department of Neurology, Medical University of Graz, Graz, Austria
1High Field MR Centre, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Karl Landsteiner Institute for Clinical Molecular MR in Musculoskeletal Imaging, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4High Field MR Centre, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 5Institute of Scientific Instruments of the CAS, Brno, Czech Republic, 6Department of Internal Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna, Vienna, Austria, 7Centre of Advanced Imaging, University of Queensland, St. Lucia, Australia, 8Department of Neurology, Medical University of Graz, Graz, Austria
Synopsis
Imaging of body regions containing a significant amount of fat is adversely affected by chemical shift artefacts. We propose a new fat-water imaging method that uses spectrally selective dual-band excitation and CAIPIRINHA to generate separate images of fat and water simultaneously as well as chemical shift-corrected, recombined fat-water images. Gradient-echo and turbo spin-echo variants of this Simultaneous Multiple Resonance Frequency Imaging (SMURF) approach yielded fat-water separation which was similar to or better than state-of-the-art techniques in the knee, breasts and abdomen and generated recombined fat-water images in which chemical shift effects were fully eliminated.
Introduction
Imaging of body regions containing fat is affected by artefacts arising from the circa 3.5 ppm1 chemical shift (CS) of fat with respect to water. This CS causes a displacement of fat relative to water along frequency-encoding direction(s) (Type 1 CS artefact). In gradient-echo imaging, the complex summation (interference) of fat and water signals, which are subject to different phase evolutions, also leads to an echo-time dependent signal cancellation in mixed voxels (Type 2 CS artefact).The Dixon method2 is the only approach which simultaneously generates separate images of fat and water. It requires a multi-echo acquisition, however, which prolongs the acquisition time, and high receiver bandwidth, resulting in poor image SNR. In addition, Dixon images often contain fat-water swaps which may lead to image misinterpretation.
Here we propose a new method, Simultaneous Multiple Resonance Frequency imaging (SMURF), in which multi-band pulses3 simultaneously but separately excite fat and water and CAIPIRINHA4,5 with parallel imaging reconstruction6,7 separate the corresponding signals. In 2D and slab-selective 3D imaging, spatial-spectral pulses8 are employed to achieve concurrent spectral and spatial selectivity and thus avoid fat-water cross-excitation between slices. The resulting fat and water images can either be considered individually, or the fat signal can be corrected for the chemical shift displacement of
$$N_{voxels}=\frac{∆f}{rBW/pixel},$$
and, for GRE acquisitions, for the phase discrepancy of
$$∆ϕ(TE)=mod(TE(\frac{1}{Δf}))2π,$$
where Δf is the chemical shift difference, prior to recombination (Figure 1). This generates fat-water images similar to those obtained with conventional broadband acquisition but free of chemical shift artefacts.
Methods
Gradient-echo and turbo spin-echo fat-water SMURF sequences were developed for a 3T Siemens PRISMA scanner. An 11.76ms least-squares filtered minimum-phase Shinnar-Le Roux pulse9,10 of BW=350Hz was designed using Vespa11,12 and used to create both bands of the dual-band pulse, which was applied in non-selective 3D excitation and also served as an envelope of the spatial-spectral pulse (Figure 2) applied in 2D acquisitions.One knee, both breasts, and the abdominal region, each of 3 volunteers, were scanned to compare SMURF with the state-of-the-art suppression and separation techniques; water-saturation (WaterSat), fat-saturation (FatSat), and several of the most commonly used Dixon methods.
Sagittal 2D TSE knee images were acquired with anterior‐posterior phase‐encoding direction, FOV=160x160mm, resolution=0.5x0.5x3mm, 36 slices, ETL=4, TR=2500ms and rBW/pixel=150Hz. SMURF and “conventional” (broadband excitation) images as well as WaterSat and FatSat images were acquired with an 11.94ms echo spacing and TA=3:17min. 2pt Dixon images were acquired using Siemens’ product sequence (“tse_dixon”) with 14ms echo spacing (the minimum possible) and TA=6:35min.
Transversal 2D GRE breast and abdominal images were acquired in single breathholds with left-right and anterior-posterior phase-encoding directions respectively, all with matrix size=320x320, resolution=1.0x1.0x3mm and PF=6/8. 6 slices of SMURF and “conventional” images and 4 slices of WaterSat and FatSat images were acquired with TE=6.8ms, TR=110ms, rBW/pixel=240Hz and the respective fat and water Ernst angles of FA(fat)=42° and FA(water)=22°. For comparison, two Dixon images were acquired: i) slab-selective 3D 2pt Dixon with 8 slices, TE={2.27,5.67}ms, TR=8ms, FA=9° and rBW/pixel=490Hz using Siemens´ product sequence (“VIBE13”); and ii) 2D 3pt Dixon with 6 slices, TE={2.2,5.3,8.4}ms, TR=110ms, FA=32° and rBW/pixel=540Hz for offline reconstruction with the graph-cut approach14 from the Fat-water Toolbox15.
Low resolution GRE scans were also acquired for B0 field mapping17 and to calculate the GRAPPA kernel for fat-water unaliasing with slice-GRAPPA5 performed in MATLAB.
Results
For all body regions and all volunteers, the magnitude of local field deviations throughout the FOV was below 220Hz and SMURF generated cleanly separated water and fat images, with minimal unaliasing artefacts or cross-excitation (Figure 3).The knee TSE SMURF images were similar to the Dixon images and to the separately acquired fat- and water-saturated images (Figure 4a). With SMURF, there was a slight increase in signal attributed to water in fatty tissues, but the acquisition time was halved.
In GRE imaging of the breasts (Figure 4b) and abdomen (Figure 4c), fat-saturated and 2pt Dixon water images showed quite high residual fat signal. The 3pt Dixon images show very little residual signal, but some swaps were present in almost all subjects. SMURF achieved very little residual signal and correct fat-water attribution in all cases.
Image contrast of the recombined SMURF fat-water images was similar to the “conventional” (broadband) images and the chemical shift artefacts were completely eliminated (Figure 5).
Discussion and Conclusion
SMURF, a new fat-water imaging method, has been presented in gradient-echo and turbo spin-echo variants and was shown to yield well-separated fat and water images and also chemical shift artefacts-free recombined fat-water images. The spectral complexity of fat, which has been neglected in SMURF, resulted in a slightly reduced fat‐water separation quality compared to the 3pt GRE and 2pt TSE Dixon. However, compared to the 2pt GRE Dixon, which is sensitive to B0 inhomogeneity and assumes no T2* decay, and to the separate acquisitions with fat- and water-saturation, SMURF yielded improved separation. Moreover, SMURF requires only one, single-echo acquisition with echo-times and receiver bandwidths which can be chosen freely and uses well-established, robust reconstruction methods.SMURF, although introduced as a fat-water imaging method, could also be used for simultaneous imaging of other chemical species, such as phosphocreatine and inorganic phosphate17 or hyperpolarized 13C pyruvate and its metabolites18,19.
Acknowledgements
This study was funded by the Austrian Science Fund (FWF): 31452. SR was additionally supported by the Marie Skłodowska-Curie Action (MS-fMRI-QSM 794298), MK by Austrian Federal Ministry of Education, Science and Research (BMWFW WTZ Mobility, CZ09-2019) and RK by the Czech Academy of Sciences (MSM100651801). The financial support by the Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development is also gratefully acknowledged.References
- Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008;49(9):2055-2062. doi:10.1194/jlr.D800010-JLR200
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189-194. doi:10.1148/radiology.153.1.6089263
- Müller S. Multifrequency selective rf pulses for multislice MR imaging. Magn Reson Med. 1988;6(3):364-371.
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53(3):684-691. doi:10.1002/mrm.20401
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. doi:10.1002/mrm.23097
- Pruessmann K, Weiger M, Scheidegger M, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952-962.
- Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210.
- Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med. 1990;15(2):287-304.
- Shinnar M, Eleff S, Subramanian H, Leigh JS. The synthesis of pulse sequences yielding arbitrary magnetization vectors. Magn Reson Med. 1989;12(1):74-80.
- Le Roux P. Exact synthesis of radio frequency waveforms. In: Proceedings of the 7th Annual Meeting of SMRM.
- Soher BJ, Semanchuk P, Todd D, Steinberg J, Young K. VeSPA: Integrated applications for RF pulse design, spectral simulation and MRS data analysis. In: Proceedings of the 19th Annual Meeting of ISMRM. Montreal, Quebec, Canada, 2011. #1410.
- https://www.opensourceimaging.org/project/vespa-versatile-simulation-pulses-analysis/. Accessed March 19, 2021.
- Rofsky NM, Lee VS, Laub G, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212(3):876-884. doi:10.1148/radiology.212.3.r99se34876
- Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust Water/Fat Separation in the Presence of Large Field Inhomogeneities Using a Graph Cut Algorithm. Magn Reson Med. 2010;63(1):79-90. doi:10.1002/mrm.22177ISMRM Fat-water toolbox.
- ISMRM fat-water separation workshop 2012. “http://www.ismrm.org/workshops/FatWater12/” Accessed March 19, 2021.
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65-73.
- Schmid AI, Meyerspeer M, Robinson SD, et al. Dynamic PCr and pH imaging of human calf muscles during exercise and recovery using 31P gradient-Echo MRI at 7 Tesla. Magn Reson Med. 2016;75(6):2324-2331. doi:10.1002/mrm.25822
- von Morze C, Reed G, Shin P, et al. Multi-band Frequency Encoding Method for Metabolic Imaging with Hyperpolarized [1-13C]Pyruvate. J Magn Reson San Diego Calif 1997. 2011;211(2):109-113. doi:10.1016/j.jmr.2011.04.007
- Larson PEZ, Kerr AB, Chen AP, et al. Multiband Excitation Pulses for Hyperpolarized 13C Dynamic Chemical Shift Imaging. J Magn Reson San Diego Calif 1997. 2008;194(1):121-127. doi:10.1016/j.jmr.2008.06.010
- Yu H, Reeder SB, Shimakawa A, Gold GE, Pelc NJ, Brittain JH. Implementation and Noise Analysis of Chemical Shift Correction for Fast Spin Echo Dixon Imaging. In: Proceedings of the 12th Annual Meeting of ISMRM, Kyoto, Japan, 2004. #2686.
Figures
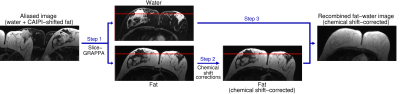
Figure 1: Fat‐water separation, chemical shift corrections, and
recombination in Simultaneous Multiple Resonance Frequency (SMURF) imaging.
Overlapping water and CAIPIRINHA‐shifted fat images are unaliased using
slice‐GRAPPA (Step 1). The fat image is shifted to reverse chemical shift
displacement – see the positions relative to the red reference line – and, for
GRE acquisitions, corrected for chemical shift‐related phase evolution (Step 2).
The fat and water images are then recombined (Step 3), generating a fat‐water
image free of chemical shift artefacts.
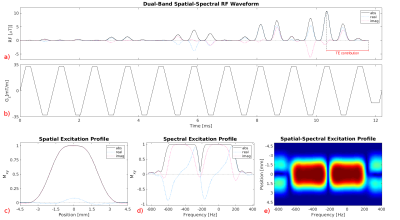
Figure 2: Dual‐band spatial‐spectral pulse used
for 90° excitation in 2D TSE‐SMURF imaging. a) RF waveform created by a train
of 0.56 ms sinc subpulses modulated by a dual‐band envelope. b) Waveform of the
oscillating trapezoidal z‐gradient played concurrently with the RF pulse for
slice‐selection. Excitation profile of the RF pulse, comprising two frequency
bands offset by 440 Hz, shown as a function of the position along the
slice‐selection direction (c), the Larmor frequency (d), and the Larmor
frequency and position along the slice‐selection direction (e).

Figure
3: Excitation selectivity and unaliasing quality of SMURF imaging demonstrated
for one exemplary volunteer for each body region under consideration. The
acquired aliased images show the overlapping water and fat images, which are CAIPIRINHA‐shifted
along the phase‐encoding (PE) direction. Separate SMURF water and fat images,
reconstructed from the aliased images, illustrate the achieved separation
quality. (The separated images were rescaled for improved visibility.)
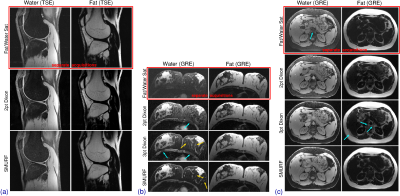
Figure
4: Comparison of the fat‐ and water‐saturated (top), Dixon (middle), and SMURF
(bottom) images. In the TSE knee imaging (a) FatSat and SMURF water images show
slightly higher residual fat signal. In breast (b) and abdominal (c) GRE imaging,
FatSat and 2pt Dixon images show high residual signal but no fat‐water swaps. The
3pt Dixon images show very little residual signal, improving the visibility of
small structures (gold arrows), but some swaps (blue arrows). SMURF images show
little residual signal and no fat‐water swaps. (The same non‐linear scales were
used for all images.)
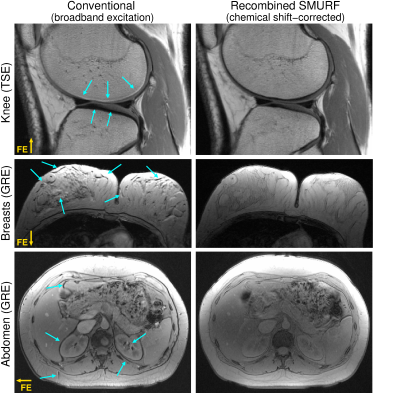
Figure
5: Comparison
of left) conventional images and right) chemical shift displacement‐corrected,
recombined SMURF fat‐water images. The SNR of the SMURF images is lower than
that of the conventional images (expected reduction by a factor of , see Ref 20), but the fat‐water misalignment is
fully eliminated. Note the slightly altered fat‐water contrast in SMURF GRE
images resulting from the use of separate Ernst angles for fat and water. (The
same non‐linear scales were used for all images.)