0115
Three-Dimensional Surface-Based Analysis of Cartilage MRI Data in Knee Osteoarthritis: Validation and Initial Clinical Application
James MacKay1,2, Joshua Kaggie1, Graham Treece3, Stephen McDonnell4, and Wasim Khan4
1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Norwich Medical School, University of East Anglia, Norwich, United Kingdom, 3Department of Engineering, University of Cambridge, Cambridge, United Kingdom, 4Department of Surgery, University of Cambridge, Cambridge, United Kingdom
1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Norwich Medical School, University of East Anglia, Norwich, United Kingdom, 3Department of Engineering, University of Cambridge, Cambridge, United Kingdom, 4Department of Surgery, University of Cambridge, Cambridge, United Kingdom
Synopsis
- Conventional MRI outcome measures for cartilage in knee osteoarthritis (OA) clinical studies lack responsiveness and require time-consuming manual analysis.
- Here we validate and clinically implement a semiautomatic surface-based approach termed 3D Cartilage Surface Mapping (3D-CaSM) which overcomes these issues.
- Validation data demonstrate comparable bias, precision, repeatability and reproducibility to expert manual segmentation (current standard) but with >10 fold reduction in analysis time.
- Clinical data indicate improved sensitivity to change in one observational and two interventional (exercise, knee joint distraction) studies.
INTRODUCTION
Traditional quantitative analysis of cartilage with MRI requires time consuming manual segmentation and averages measurements (e.g., thickness) across regions of interest (ROIs) which may reduce responsiveness1.METHODS
The described work consists of a validation study in cadavers (referred to as the validation study) and three in-vivo studies demonstrating the clinical application of this method (referred to as the clinical studies).The validation study compared cartilage thickness measurements performed on embalmed cadaveric knees (n = 4) between MRI and the reference method of high resolution peripheral quantitative computed tomography (HRpQCT). The first clinical study assessed the inter-observer reproducibility and test-retest repeatability of 3D-CaSM and sensitivity to change over six months participants (n = 14) with knee osteoarthritis (OA) and 6 age-matched healthy volunteers. Subsequent clinical studies explored the utility of 3D-CaSM for (1) assessing changes in cartilage composition (T2, T1rho) in response to exercise in 19 healthy knees and (2) assessing changes in cartilage thickness in response to knee joint distraction (KJD) treatment in 20 patients with knee OA.
MRI cartilage thickness measurements were performed using 3D spoiled gradient recalled echo (SPGR) sequences with fat suppression; MRI cartilage compositional measurements were performed using T1rho/T2 magnetization prepared pseudo-steady-state 3D fast spin echo (FSE) sequences2. Typical sequence parameters are provided in Figure 1.
The initial 3D-CaSM analysis process is summarized in Figure 2a. This results in approximately 6000 thickness measurements for a single knee, together with accurately located inner and outer cartilage surfaces. These surfaces can then be imported into compositional maps following appropriate image registration (Figure 2b) to generate a corresponding set of compositional measurements. Intra-individual and inter-individual spatially corresponded analysis is facilitated by surface-to-surface registration to a canonical (‘average’) surface using a combined similarity/thin plate spline algorithm. All analyses are performed using freely available software; Stradview (http://mi.eng.cam.ac.uk/Main/StradView/) for 3D-CaSM and wxRegSurf (http://mi.eng.cam.ac.uk/~ahg/wxRegSurf/) for surface-to-surface registration. For the validation study, for each cadaveric knee a set of corresponding HRpQCT thickness values (resolution 0.08mm isotropic) from disarticulated knees was obtained and compared with 3D-CaSM and expert manual segmentation of the MRI data using Bland-Altman analysis.
For the clinical studies, Bland-Altman analysis and root-mean-square coefficients of variation (RMS-CVs) were used for assessment of test-retest repeatability and inter-observer reproducibility. Responsiveness to change was assessed at the individual level via calculation of the percentage of each cartilage surface affected by areas of significant change (%SC), defined using thresholds based on area and smallest detectable difference (SDD), and at the group level using statistical parametric mapping (SPM).
RESULTS
3D-CaSM reduces analysis time for a single knee by >10 fold compared to conventional manual segmentation (15 minutes vs 3 hours). Agreement for cartilage thickness measurement with gold-standard HRpQCT was good (mean bias [95% limits of agreement] = 0.06 [-0.43,0.56] mm) and similar to expert manual segmentation (-0.13 [-0.64,0.38] mm).Inter-observer RMSCVs were similar for 3D-CaSM and manual segmentation and test-retest repeatability RMSCVs were <10% in all cases (Figure 3). In the observational clinical study, the number of participants demonstrating significant changes in cartilage thickness and/or composition at 6 months was 13/14 with 3D-CaSM vs 1/14 using standard methods. Use in interventional studies has allowed identification of focal regions of statistically significant change in cartilage in response to exercise (Figure 4) and following knee joint distraction not detectable by standard methods (Figure 5).
CONCLUSION
3D-CaSM is a valid tool to assess changes in cartilage morphology and composition over durations relevant to both observational and interventional clinical studies in OA. Bias, precision, test-retest repeatability and inter-observer reproducibility are comparable to current gold-standard methods. Improved responsiveness of 3D-CaSM could permit smaller, shorter duration clinical trials. Work is ongoing to assess association with symptomatic progression in two large cohort studies (APPROACH & PROGRESS-OA)3,4.Acknowledgements
No acknowledgement found.References
- Jørgensen DR, Lillholm M, Genant HK, Dam EB: On Subregional Analysis of Cartilage Loss from Knee MRI. Cartilage 2013; 4:121–130.
- Chen W, Takahashi A, Han E: 3D Quantitative Imaging of T1rho and T2. In Proc Intl Soc Mag Reson Med. Volume 19. Montreal; 2011:231.
- Helvoort EM van, Spil WE van, Jansen MP, et al.: Cohort profile: The Applied Public-Private Research enabling OsteoArthritis Clinical Headway (IMI-APPROACH) study: a 2-year, European, cohort study to describe, validate and predict phenotypes of osteoarthritis using clinical, imaging and biochemical markers. BMJ Open 2020; 10:e035101.
- Biomarkers Consortium - PROGRESS OA: Clinical Evaluation and Qualification of Osteoarthritis Biomarkers | FNIH [https://fnih.org/what-we-do/biomarkers-consortium/programs/progress-oa]
Figures
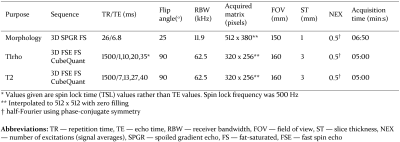
Figure 1: Typical MR pulse sequence parameters for 3D-CaSM
analysis (based on GE750 platform [3T])
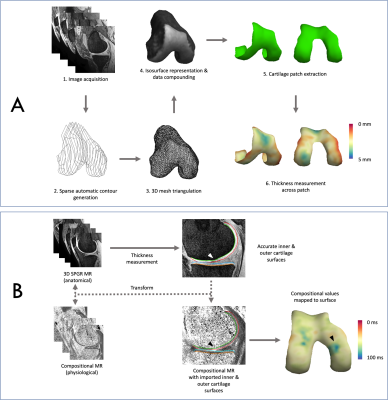
Figure 2: asdf
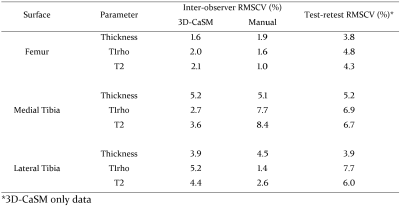
Figure 3: Summary of reproducibility (inter-observer) and
repeatability (test-retest) data for 3D-CaSM and expert manual segmentation
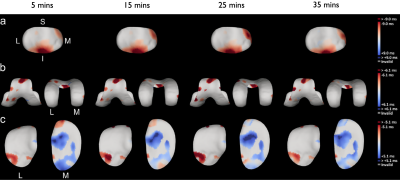
Figure 4: Implementation of 3D-CaSM in a study of changes in
cartilage composition (T1rho in this example) in 19 healthy participants
following an exercise task for patellar (top row, a), femoral (middle row, b) & tibial (bottom row, c) cartilage surfaces. Surface data displayed represent average change from pre-exercise scan,
ordered left to right by timepoint & masked according to smallest detectable
difference (derived from test-retest data). Consistent subregional reductions
in T1rho in the inferior patella & superior trochlea & increased in the
medial tibial plateau are seen.
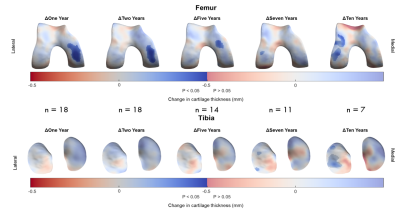
Figure 5: Implementation of 3D-CaSM in a clinical study of knee
joint distraction using statistical parametric mapping to detect effects at a
group level. Surface data represent average change from baseline mapped to
canonical surface and masked according to statistical significance. Significant
increases in cartilage thickness are seen over the weight-bearing medial
femoral condyle at up to 5 years follow-up, with a possible delayed
compensatory response in the lateral compartment at 7 years & 10 years
follow-up.