0112
Combined spin echo and gradient echo slice-to-volume reconstruction in fetal diffusion MRI
Daan Christiaens1,2, Maximilian Pietsch1, Lucilio Cordero-Grande1, Anthony N Price1,3, Jana Hutter1,3, Emer Hughes1, Serena J Counsell1, Joseph V Hajnal1,3, and J-Donald Tournier1,3
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Electrical Engineering (ESAT-PSI), KU Leuven, Leuven, Belgium, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Electrical Engineering (ESAT-PSI), KU Leuven, Leuven, Belgium, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Modelling tissue microstructure during fetal and neonatal brain development relies on robustly sampling diverse, low-SNR image contrast in moving subjects. Here, we extend slice-to-volume reconstruction to multi-echo diffusion MRI, in order to correct subject motion across the combined diffusion-T2*-weighted signal. Our results suggest that the spin echo and gradient echo dMRI signal have different trends across gestational age. Tissue microstructure models could therefore leverage these multidimensional data to reveal new insights in brain development.
Introduction
Fetal diffusion MRI can probe tissue microstructure during the critical phase of early brain development in the third trimester, and hence contribute to a better understanding and diagnosis of neuropsychiatric disorders. However, the relatively low SNR in abdominal imaging limits the maximum achievable b-value and hence the range of contrasts that can be sampled with conventional diffusion MRI. Furthermore, subject motion and dynamic EPI distortion are considerable challenges that need to be addressed in data processing.The Developing Human Connectome Project (dHCP) has therefore developed a closely integrated acquisition and analysis pipeline for fetal diffusion MRI at 3T, which incorporates denoising, dynamic distortion correction and slice-to-volume reconstruction (SVR) 1. Of particular interest here, to deal with dynamic distortion correction, two echoes were acquired in the fetal dHCP protocol using a combined spin echo and field echo (SAFE) sequence 2. So far, the second echo has been used only for distortion correction, but is likely to contain useful additional information, as suggested by recent developments in the field of multidimensional diffusion MRI 3,4. Here, we extend the SVR to reconstruct combined diffusion-T2*-weighted images, offering a wider range of contrasts to be leveraged in quantitative tissue modelling.
Methods
Data. Fetal imaging data were acquired in 264 subjects between 21 and 38 weeks postmenstrual age (wPMA). All scanning was done on a 3T Philips Achieva system using a 32 channel cardiac receiver coil 5. dMRI data were acquired with a two-shell combined spin echo and field echo (SAFE) sequence (Figure 1). Data are pre-processed with denoising in the complex signal domain 6. A dynamic B0 field map, estimated from the phase difference between the spin and gradient echos, is used for distortion correction 2.Slice-to-volume reconstruction. Subject motion is assumed to be frozen during the acquisition time of each multi-band excitation, or otherwise lead to signal dropout in either or both the spin and gradient echo. Therefore, SVR simultaneously estimates motion parameters for each excitation, outlier weights for each echo, and a representation of the motion-corrected multi-shell dMRI signal. We build on the SHARD-SVR framework 7 previously demonstrated in fetal dMRI to estimate the motion parameters and reconstruct the spin echo data. Then we reconstruct the gradient echo data with shared motion parameters but independent slice outlier weights.
Analysis. Explorative analysis of the spin echo and gradient echo signal in the parenchyma was based on age trends in the fibre response function. This analysis was done on a subset of 197 of cases with high quality SVR. A set of single-fibre voxels were estimated based on the spin echo b=1000s/mm2 shell and then used to derive the mean signal response per subject 8, which are subsequently averaged in bi-weekly bins.
Results and Discussion
Figure 2 shows an example of the shell mean before and after SVR in a case with severe motion (86-percentile rank rotation). SVR has resolved motion-induced blurring and slice dropouts in all shells and in spin echo and gradient echo contrasts, with mutual spatial alignment between them. The corresponding motion trace and slice weights are shown in Figure 3. The slice weights of corresponding spin echo and gradient echo slices are significantly correlated in all subjects with median Pearson correlation coefficient R=0.765.Figure 4 explores global trends in the signal attenuation and anisotropy in the estimated response function across fetal brain development. The spin echo response confirms slower attenuation and increasing anisotropy with gestational age, in line with previous findings in neonates 9. However, the gradient echo response suggests a different relationship with gestational age, most prominently at b=0. This suggests that the gradient echo contrast may bring valuable additional information that could inform future models of tissue microstructure in the developing brain.
Conclusion
Combined spin echo and gradient echo dMRI facilitates dynamic distortion correction, but can also offer additional contrast for tissue modelling in a regime where high b-values may be out of reach. Future work will explore more advanced models to exploit the additional information contained within these multi-dimensional diffusion data in the fetal brain.Acknowledgements
DC is supported by the Flemish Research Foundation (FWO; grant number 12ZV420N). This work was further supported by ERC grant agreement no. 319456 (dHCP project), by the Wellcome EPSRC Centre for Medical Engineering at King’s College London [WT 203148/Z/16/Z], and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
- Christiaens D, Cordero-Grande L, Price AN, Hutter J, Hughes E, Counsell SJ, Tournier J-D, Hajnal JV. Fetal diffusion MRI acquisition and analysis in the developing Human Connectome Project. ISMRM 2019; 27:O629
- Cordero-Grande L, Price AN, Ferrazzi G, Hutter J, Christiaens D, Hughes E, Hajnal JV. Spin And Field Echo (SAFE) dynamic field correction in 3T fetal EPI. ISMRM 2018; 26:O208.
- Topgaard D. Multidimensional diffusion MRI. J Magn Reson 2017; 275:98-113.
- Hutter J, Slator PJ, Christiaens D, Teixeira R, Roberts T, Jackson L, Price AN, Malik S, Hajnal JV. Integrated and efficient diffusion-relaxometry using ZEBRA. Scientific Reports 2018; 8:15138.
- Price AN, Cordero-Grande L, Hughes E, [...], Edwards, AD, Hajnal JV. The developing Human Connectome Project (dHCP): fetal acquisition protocol. ISMRM 2019; 27:P244.
- Cordero-Grande L, Christiaens D, Hutter J, Price AN, Hajnal JV. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage 2019; 200:391-404.
- Christiaens D, Cordero-Grande L, Pietsch M, Hutter J, Price AN, Hughes EJ, Vecchiato K, Deprez M, Edwards AD, Hajnal JV, Tournier J-D. Multi-shell SHARD reconstruction from scattered slice diffusion MRI data in the neonatal brain. NeuroImage 2021; 225:117437.
- Tournier J-D, Calamante F, Connelly A. Determination of the appropriate b-value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed 2013; 26(12):1775-86.
- Pietsch M, Christiaens D, Hutter J, Cordero-Grande L, Price AN, Hughes E, Edwards AD, Hajnal JV, Counsell SJ, Tournier J-D. A framework for multi-component analysis of diffusion MRI data over the neonatal period. NeuroImage 2019; 186:321-337.
Figures
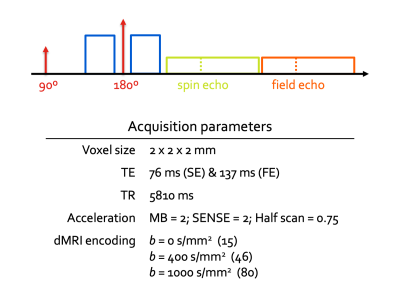
Figure 1: Structure and parameters of the spin and field echo (SAFE) sequence. The standard PGSE is extended with a second (T2*-weighted) readout. The phase difference between both echos is used to estimate the instantaneous B0 field map.
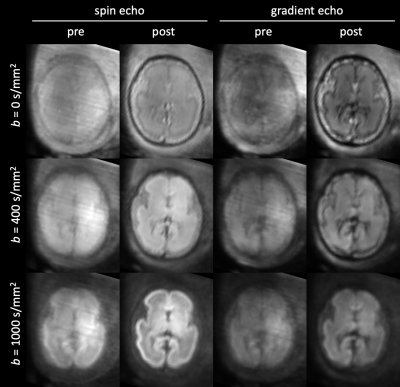
Figure 2: Example of slice-to-volume reconstruction (SVR) in a fetus (28 wPMA) with severe head motion. The images show the mean of each shell before and after SVR, in the spin echo and gradient echo contrast. Grayscale values are min-max normalised per row.
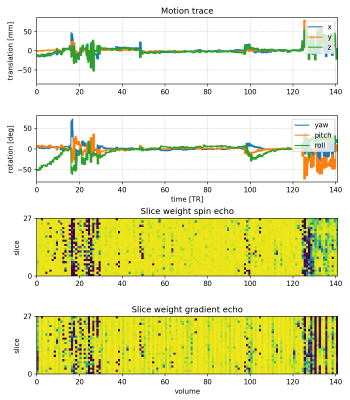
Figure 3: Estimated subject motion trace and slice weights of the fetal scan shown in Figure 2. SVR resolved motion in the range of +/- 50 deg. The slice weights (blue: outlier, yellow: inlier) of the spin and gradient echo images are strongly correlated and also correlate with the motion traces over time.
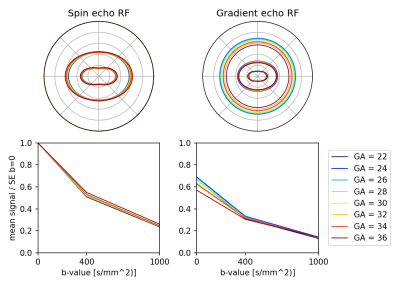
Figure 4: Age trends in the mean tissue response function (RF) in the parenchyma. Tissue RFs for each subject were averaged across bi-weekly age intervals. The spin echo RF shows increasing signal anisotropy and stronger attenuation at young gestational age, as expected. On the other hand, the gradient echo shows stronger T2*-weighting at later gestational ages, most prominently in the b=0 signal.