0110
SNR efficiency and effectiveness of 7T high-b diffusion imaging with MESMERISED and PGSE1Department of Cognitive Neuroscience, Faculty of Psychology & Neuroscience, Maastricht University, Maastricht, Netherlands, 2Department of Systems Neurosciences, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, Hamburg, Germany
Synopsis
We compare MESMERISED and standard PGSE for their signal-to-noise efficiency in b-value = 7000 s/mm2 (b7k) dMRI at 7T and their effectiveness in supporting microstructure modeling. At current state-of-the-art gradient performance, MESMERISED’s SAR and gradient duty cycle efficiency allows it to outperform PGSE for 7T high-b dMRI and provide highly efficient and effective microstructure modeling. Its added capacity to explore diffusion times, super-accelerate quantitative T1 mapping, and also perform qT2 and B1+ mapping, makes it highly useful for quantitative multi-contrast and diffusion MRI.
Introduction
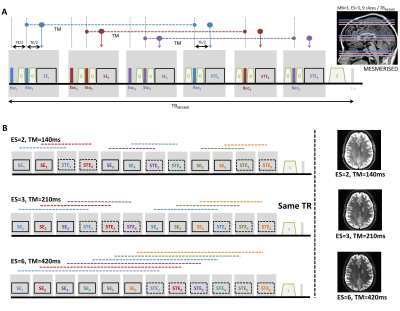
Microstructure modeling with diffusion MRI (dMRI) is steadily requiring more richly sampled data and higher b-values. Two of the prominent developments that have supported these needs are higher amplitude gradient systems and simultaneous multi-slice (a.k.a. multi-band) imaging1,2,3. Although the increased baseline signal at 7T has been employed for high spatial resolution dMRI at moderate b-values4, the decreased T2 has dampened the interest in high-b dMRI at 7T. We have introduced MESMERISED (Figure 1) for super-accelerated STEAM imaging of quantitative T1 mapping and dMRI achieving high b-values and/or long diffusion times at 7T5. Here, we compare MESMERISED and standard pulsed-gradient spin echo (PGSE) for their signal-to-noise efficiency in b-value = 7000 s/mm2 (b7k) dMRI at 7T and their effectiveness in supporting microstructure modeling.Methods
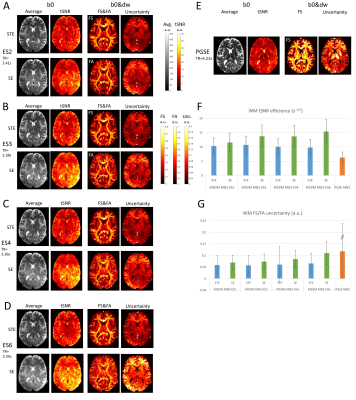
For objective comparison between MESMERISED and PGSE, the same MESMERISED-capable sequence was run in PGSE mode (no STE block, modified storing pulse phase and flip angle of 180 degrees). Whole brain (72 slices) 1.8mm isotropic b7k diffusion-weighted MESMERISED (ESxMB: 2x3//3x3//4x3//6x3; TR: 3.40//3.37//3.36//3.35 s.; TE: 49.9//46.2;//43.8//40.9 ms; TM: 140//210//280//420 ms; G-diff: 70 mT/m; SE b-val: 752//483//350//223 s/mm2) and PGSE (MB: 2; TR: 4.21 s.; TE: 97.8 s.; G-diff: 49mT/m) were optimized to run just within SAR and gradient limits. This allowed full acquisition duty cycle and minimal TR for MESMERISED, but gradient limits forced b7k PGSE to run at Gdiff=49mT/m lengthening TR and TE. EPI readout (GRAPPA-2 with FLEET ACS, FoV 218x218mm2 and PF 6/8) was matched for echo-spacing (0.57 ms) and for bandwidth within 5% (MESMERISED: 2164 Hz/pix; PGSE: 2066 Hz/pix). Both MESMERISED and PGSE protocols ran close to the 100% 6-minute SAR limit. For each, 60 directions at b7k and 6 b0’s as well as 27 matched b0s were acquired in one healthy subject (male, 79 kg) on a Magnetom Siemens 7T human MRI system (Siemens Healthineers, Erlangen, Germany). All data was distortion corrected with Topup and Eddy, FSL v6.0.16, analyzed using MDT7 with Tensor (66 MESMERISED-SE volumes) and CHARMEDr2 (66 MESMERISED-STE volumes 66 PGSE volumes) diffusion models. Temporal signal-to-noise ratio (tSNR=mean/std) and tSNR efficiency (tSNR-eff = tSNR/√effTR, where effTR=TR/2 for STE and SE in MESMERISED) were calculated from the 27 b0 volumes. Uncertainty in the fractional anisotropy (FA, from Tensor) and fraction-of-sticks (FS, from CHARMEDr2) estimation was derived from the Fisher Information Matrix as previously described8.Results
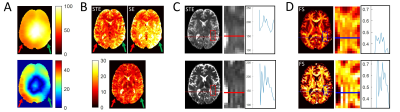
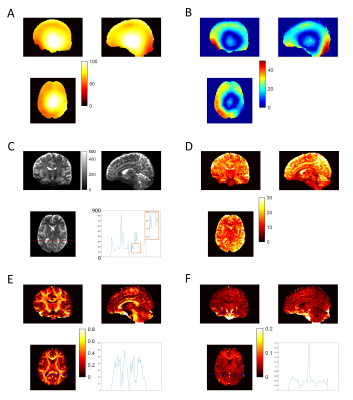
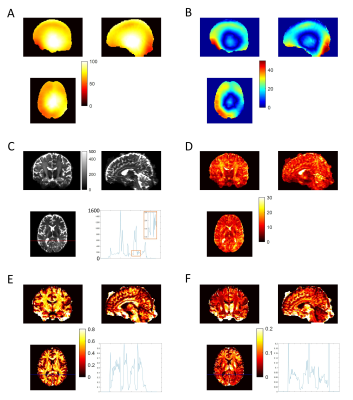
White matter (WM) b0 tSNR-eff is markedly higher for both STE and SE volumes of all MESMERISED acquisitions than for PGSE (Fig. 2F). MESMERISED-STE tSNR-eff is at a stable level for all ES (2,3,4,6) while MESMERISED-SE tSNR-eff increases with ES (and decreasing TE). MESMERISED’s main efficiency advantage is through the acquisition of both STE and SE volumes in a shorter TR. WM FS uncertainty is markedly lower for MESMERISED-STE volumes than for PGSE, and WM FA uncertainty is low for MESMERISED-SE volumes for ES=2,3,4 (the higher ES=6 values reflect the low b= 223 s/mm2). FR for PGSE additionally lacks WM contrast and appears to have an upward bias. tSNR and uncertainty appear negatively associated and driven by B1+ inhomogeneity (Fig. 3A). Areas with a similar distance to receiver RF-coils, but with high (red arrow) and low (green arrow) flip angle deviation have low and high tSNR, respectively (Fig. 3B), while overall tSNR appears higher in MESMERISED. This pattern is evident over the whole brain (Fig. 4&5). An additional factor in the lower FS uncertainty for PGSE is likely Gibbs ringing caused by the high water signal through a long TE and excessive T2-weighting (Fig. 5C vs 4C), which is not present in MESMERISED (Fig. 3C&D).Discussion
MESMERISED displays markedly higher tSNR-eff than PGSE at 7T with a high performing 70mT/m gradient when matched for both readout EPI train and image reconstruction. The main factors are the limitations on SAR and gradient duty cycle, which forced PGSE to run at increased TR and TE. MESMERISED avoids these limitations through a lower SAR and gradient load (allowing full acquisition duty cycle), and the acquisition of both a high-b STE and low-b SE volume in a TR of ~3.4 s. SAR for PGSE could be improved through pTX and optimized MB pulses (e.g. PINS9, multi-PINS10, pTX-MB11,12), but the improved SAR would equally allow higher MB factors in MESMERISED. Gradient limits in PGSE could be improved by further gradient hardware developments, since higher amplitudes at high duty cycle could strongly reduce its TE (which is dominated by diffusion gradient length), while the already short TEs in MESMERISED might benefit less. On the other hand, rapid readout strategies, such as center-out spiral sampling13, could strongly reduce TE in MESMERISED (which is dominated by readout train length), and provide proportionally more benefit than for PGSE. In conclusion, at current state-of-the-art gradient performance, MESMERISED’s SAR and gradient duty cycle efficiency allows it to outperform PGSE for 7T high-b dMRI and provide highly efficient and effective microstructure modeling. Its added capacity to explore diffusion times, super-accelerate quantitative T1 mapping, and also perform qT2 and B1+ mapping, makes it highly useful for quantitative multi-contrast and diffusion MRI.Acknowledgements
No acknowledgement found.References
1. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic resonance in medicine, 2011; 67(5): 1210-1224.
2. Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS ONE, 2010; 5(12): e15710
3. Moeller, S., Yacoub, E., Olman, C.A., Auerbach, E., Strupp, J., Harel, N., Ugurbil, K., 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 63, 1144-1153.
4. Vu, A.T., Auerbach, E., Lenglet, C., Moeller, S., Sotiropoulos, S.N., Jbabdi, S., Andersson, J., Yacoub, E., Ugurbil, K., 2015. High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. Neuroimage 122, 318-331.
5. Fritz FJ, Poser BA and Roebroeck A. MESMERISED: Super-accelerated 7 T STEAM imaging for quantitative multi-contrast and diffusion MRI. bioRxiv 2020.05.15.098269
6. Jenkinson M, et al. FSL. NeuroImage, 2012; 62:782-90.8.
7. Harms, R.L., Fritz, F.J., Tobisch, A., Goebel, R., Roebroeck, A. Robust and fast nonlinear optimization of diffusion MRI microstructure models. Neuroimage, 2017; 155, 82-96
8. Harms, R.L., Fritz, F.J., Schoenmakers, S., Roebroeck, A., 2019. Fast quantification of uncertainty in non-linear diffusion MRI models for artifact detection and more power in group studies. bioRxiv, 651547.
9. Norris D. G., Koopmans P. J., Boyacioğlu R. and Barth M. Power independent of number of slices (PINS) radiofrequency pulses for low‐power simultaneous multislice excitation. Magnetic resonance in medicine, 2011; 66: 1234-1240
10. Eichner, C., Wald, L.L., Setsompop, K., 2014. A low power radiofrequency pulse for simultaneous multislice excitation and refocusing. Magn Reson Med 72, 949–958.
11. Poser, B.A., Anderson, R.J., Guerin, B., Setsompop, K., Deng, W., Mareyam, A., Serano, P., Wald, L.L., Stenger, V.A., 2014. Simultaneous multislice excitation by parallel transmission. Magn Reson Med 71, 1416–1427.
12. Wu, X., Schmitter, S., Auerbach, E.J., Moeller, S., Ugurbil, K., Van de Moortele, P.F., 2013. Simultaneous multislice multiband parallel radiofrequency excitation with independent slice-specific transmit B1 homogenization. Magn Reson Med 70, 630–638
13. Block, K.T. and Frahm, J. (2005), Spiral imaging: A critical appraisal. J. Magn. Reson. Imaging, 21: 657-668.
Figures