0106
Diffusion-Prepared Fast Spin Echo for Artifact-free Spinal Cord Imaging1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Sagittal diffusion and perfusion magnetic resonance imaging can provide unique contrast relevant to changes in the acute spinal cord injury. However, sagittal diffusion imaging with echo planar imaging often results in severe motion and susceptibility artifacts. To improve the quality of imaging, we propose a higher-order diffusion preparation combined with a fast spin echo readout. The proposed method demonstrates high quality imaging free from artifacts. Further optimization achieved more accurate diffusivity measurements independent from direction of the cord. Lastly, we show matching diffusion and perfusion maps of the injured cord, highlighting clear spatial differences in microstructure and vasculature injuries.
Introduction
Diffusion weighted magnetic resonance imaging (DWI) has shown potential for improved diagnosis and prognosis of spinal cord injury (SCI) severity. However, DWI of the spinal cord injury is often complicated by motion, susceptibility artifacts and edema at the lesion. In addition to motion, susceptibility artifacts in echo planar imaging (EPI) at high-field preclinical imaging has limited DWI to axial plane imaging. To improve image quality and allow sagittal plane imaging, we optimized a diffusion-preparation (DWprep) based on a BIR-4 design and motion compensation combined with a centric fast spin echo (FSE) readout to minimize susceptibility and motion artifacts. The artifact-free sagittal diffusion images were applied to contusion SCI rodent model and further compared to pseudocontinuous arterial spin labelling (pCASL) of the spinal cord with the identical FSE readout to reveal mismatch of microstructural and vascular damage in acute SCI.Methods
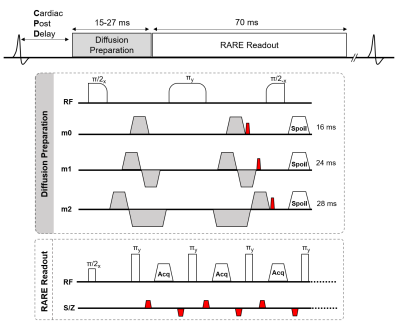
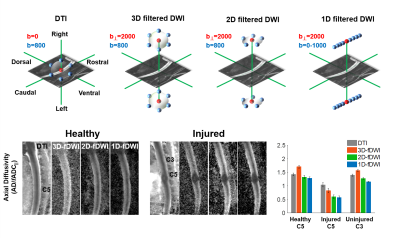
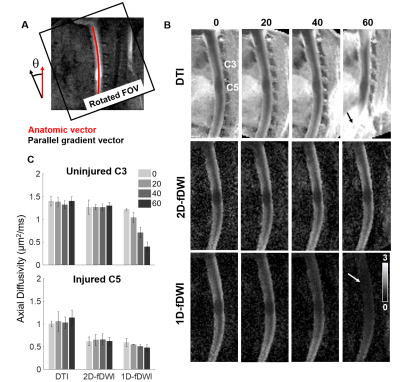
Animals: Twenty-three Sprague-Dawley rats (8- to 12-week-old, 275-300g) were used. Eight rats underwent a cervical spinal cord contusion injury. Using an NYU-MASCIC impactor, a 10-gram weight with a 2.5 mm diameter tip was dropped from a height of 12.5 mm onto the midline of the cord at C5.Magnetic Resonance Imaging Acquisition: MRI was performed on a 9.4T Bruker Biospec with a 38mm diameter Litz volume coil (Doty Scientific, Inc). A diffusion preparation used adiabatic BIR-4 pulses and zeroth through second order motion compensation encoding (MCE) diffusion gradients (Fig.1). Single slice imaging parameters of the diffusion-prepared RARE (DWprep-RARE) acquisitions were: TR/TE = 2000/4.86 ms, NEX = 1, RARE factor = 16, FOV = 30x30 mm, matrix = 96x96, slice thickness = 2 mm, bandwidth = 50 kHz, and fat saturation. For an initial demonstration of EPI artifacts, a 4-shot EPI was performed with identical special resolution using a single sagittal slice with TR/TE = 2000/30.9ms, NEX = 4, Δ = 14.44ms, and δ = 4.7ms. Prospective respiratory and cardiac gating were evaluated. Additionally, spinal cord-specific diffusion weighting schemes (Fig.3) were compared to a 25-direction scheme typical for DTI estimation with b=800 s/mm2. The schemes explicitly used a high-strength (b = 2000 s/mm2) diffusion gradient perpendicular to the cord (filter) with different sampling strategies of the other diffusion gradients in a 3D, 2D, or 1D design. These schemes were additionally rotated from nearly parallel (0˚) to 20, 40, and 60 º, relative to the spinal cord to assess orientation dependence. Perfusion imaging was acquired using pCASL labeling for the spinal cord in one healthy and three injured animals. TR/TE = 4054/5.63 ms, RARE factor = 16, FOV = 30x30 mm, matrix = 192x192, slice thickness = 2 mm, bandwidth = 70 kHz, number of repeats = 6, labeling duration = 1100 ms, and post delay of 200 ms.
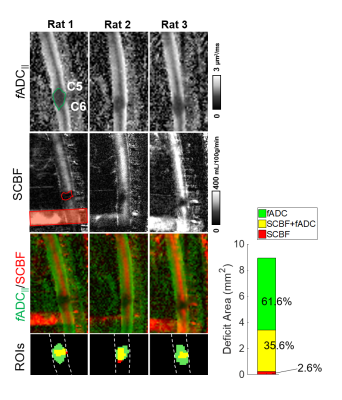
Data Analysis: The diffusion tensor and filter-probe indices were computed as previously described1 to derive axial diffusivity (AD) or filtered parallel ADC (fADC||). The maps of spinal cord blood flow (SCBF) were calculated as described previously2,3. Regions of interest (ROI) were drawn manually both in the entire cord for signal-to-noise-ratio measurements and in healthy and injured regions for comparisons of fADC||. Estimates of fADC|| and SCBF lesion overlap were calculated by manual regions of interests.
Results
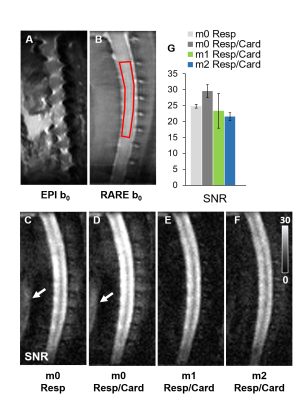
While 4-shot EPI had prominent artifacts, DWprep-RARE readout had minimal susceptibility artifacts (Fig.2A,B). Although second order (m2) DWprep slightly decreased SNR due to longer diffusion preparation (TEprep), it had the most stable SNR measurement compared to first order (m1) DWprep (Fig.2G), and both respiratory and cardiac gating was necessary in combination with m2 to minimize motion artifacts. Whereas AD from DTI did not exhibit high lesion contrast, all three filtered DWI showed higher lesion contrast. The 2D filtered encoding scheme displayed the best lesion contrast. The single-axis (1D) scheme was dependent on orientation, and the 3D scheme had lower SNR (Fig.3). A diffusion-perfusion mismatch was evident in the acutely injured spinal cord and both contrasts displayed clear lesion borders. The diffusion abnormality was approximately 3 times larger than the perfusion deficits in the same animals (Fig.5).Discussion
We systematically demonstrated that DWprep-RARE with motion compensation, dual respiratory and cardiac gating, and spinal-cord optimized filtered DW encoding provided artifact-free maps of diffusivity that clearly reveal an acute SCI lesion. These optimizations allowed a direct comparison of diffusion and perfusion imaging and revealed a clear mismatch of the lesion after experimental acute contusion SCI. Diffusion lesions occupied a substantially larger region than perfusion deficits, which may reflect different pathological mechanisms of mechanical insult. One implication of this pattern is that the areas with ongoing cytotoxic edema or axonal injury may be more available to pharmacologic treatment given the relatively preserved perfusion status. Similar examinations are needed in acute human SCI to evaluate the role of diffusion and perfusion contrast in diagnosis and prognosis.Conclusion
In this study, we used a diffusion prepared fast spin echo sequence to minimize artifacts in the rodent spinal cord. Motion compensation, prospective gating, and different filtered-DWI schemes were evaluated to identify optimal conditions for high-quality sagittal images. It is anticipated that the success of these sequences may improve the diagnosis and prognosis of traumatic SCI and show potential for clinical translation.Acknowledgements
This work was supported by funding from the National Institutes of Neurological Disorders and Stroke (R01NS109090). The authors thank Matthew Runquist and Qian (Kathleen) Yin (Medical College of Wisconsin) for experimental assistance.References
1. Skinner, N. P., Kurpad, S. N., Schmit, B. D., Tugan Muftuler, L. & Budde, M. D. Rapid in vivo detection of rat spinal cord injury with double-diffusion-encoded magnetic resonance spectroscopy. Magn. Reson. Med. 77, 1639–1649 (2017).
2. Briana Meyer; Lydiane Hirschler; Jan Warnking; Emmanuel Barbier; Matthew Budde. Preclinical Spinal Cord Perfusion Imaging with Pseudo-Continuous Arterial Spin Labeling. ISRMR SMRT Virtual Conf. (2020).
3. Buxton, R. B. et al. A general kinetic model for quantitative
perfusion imaging with arterial spin labeling. Magn. Reson. Med. 40,
383–396 (1998).
Figures